For decades, non-steroidal anti-inflammatory drugs (NSAIDs) have been the cornerstone of pain management for arthritic conditions and several other types of musculoskeletal pain.
These drugs are also frequently used to relieve symptoms of headaches, painful periods, colds, and flu.

The term nonsteroidal distinguishes NSAIDs from glucocorticoids (steroids), which are potent drugs used to treat numerous inflammatory conditions.
Apart from alleviating pain, NSAIDs reduce inflammation, and decrease fever.
NSAIDs were introduced in the 1960s and soon became the most widely prescribed class of drugs in the world, with more than 100 million prescriptions issued annually in the United States alone (1). More than 17 million Americans use NSAIDs daily.
Despite the popularity and frequent use of these agents, side effects are not uncommon, and frequently involve the gastrointestinal tract, the cardiovascular system, and the kidneys.
Indeed, the gastrointestinal toxicities associated with widespread NSAID use have been a major drawback during long term therapy.
Examples of cardiovascular and renal problems include hypertension, edema, heart attack (myocardial infarction), and impaired kidney function. This is particularly important in patients with a history of heart disease or renal disorders.
Furthermore, NSAIDs can interact with numerous other drugs which in some cases may have serious consequences.
A Brief History
Inflammation is a part of a complex biological response of the body to harmful stimuli. The classical signs of acute inflammation are pain (dolor), heat (calor), redness (rubor), swelling (tumor), and loss of function (functio laesa)(2).
It is believed that the Greek physician, Hippocrates, prescribed an extract from willow bark to treat inflammation already in the year 400 B.C. However, it took more than two thousand years before the active ingredient of willow bark, salicin, was identified. Salicin has powerful pain-relieving and anti-inflammatory effects.
In 1828, Joseph Buchner, professor of pharmacy at Munich University, Germany managed to extract salicin from willow (3).
Following the work of another German scientist, Felix Hoffman, acetylsalicylic acid (aspirin) the more palatable form of salicylic acid was introduced into the market by Bayer in 1899 (4).
Thus, aspirin is one of the oldest and most commonly used drugs to relieve pain and cure fever and is regarded as the first NSAID drug. However, the mechanisms behind its pain-relieving and anti-inflammatory effects remained elusive until the 1960s.
Sir John Robert Vane, a British pharmacologist, was instrumental in the understanding of how aspirin works. He initially suggested that aspirin might work by blocking the synthesis of prostaglandins from arachidonic acid. Prostaglandins are biochemical compounds that influence blood pressure, body temperature, allergic reactions, inflammation, and other physiological phenomena.
Together with two Swedish scientists, Sune K. Bergström and Bengt Ingemar Samuelsson, Vane won the Nobel Prize for Physiology or Medicine in 1982 for the isolation, identification, and analysis of prostaglandins.
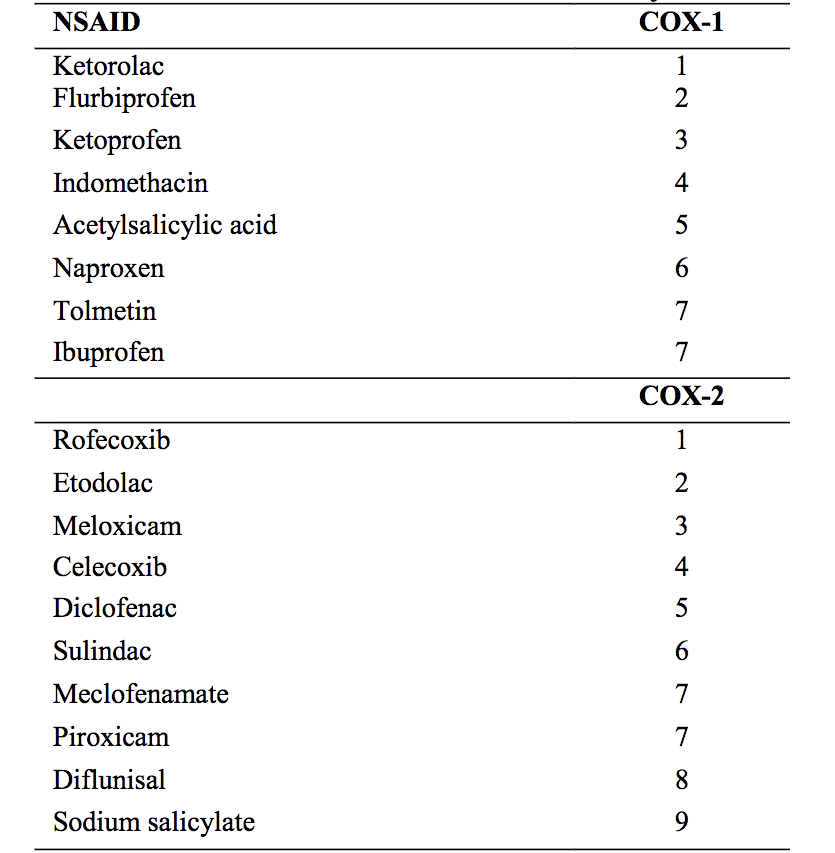
Following the discoveries of Vane and other researchers, it was found that aspirin and other NSAIDs work by inhibiting a key enzyme in prostaglandin synthesis called cyclooxygenase (COX). Later, it was discovered that there are two COX enzymes, COX-1 and COX-2.
NSAIDs that selectively inhibit COX-2 were developed in the 1990s. The main benefit of these drugs compared to other NSAIDs is that they are less likely to have gastrointestinal side effects.
How Do NSAID’s Work?
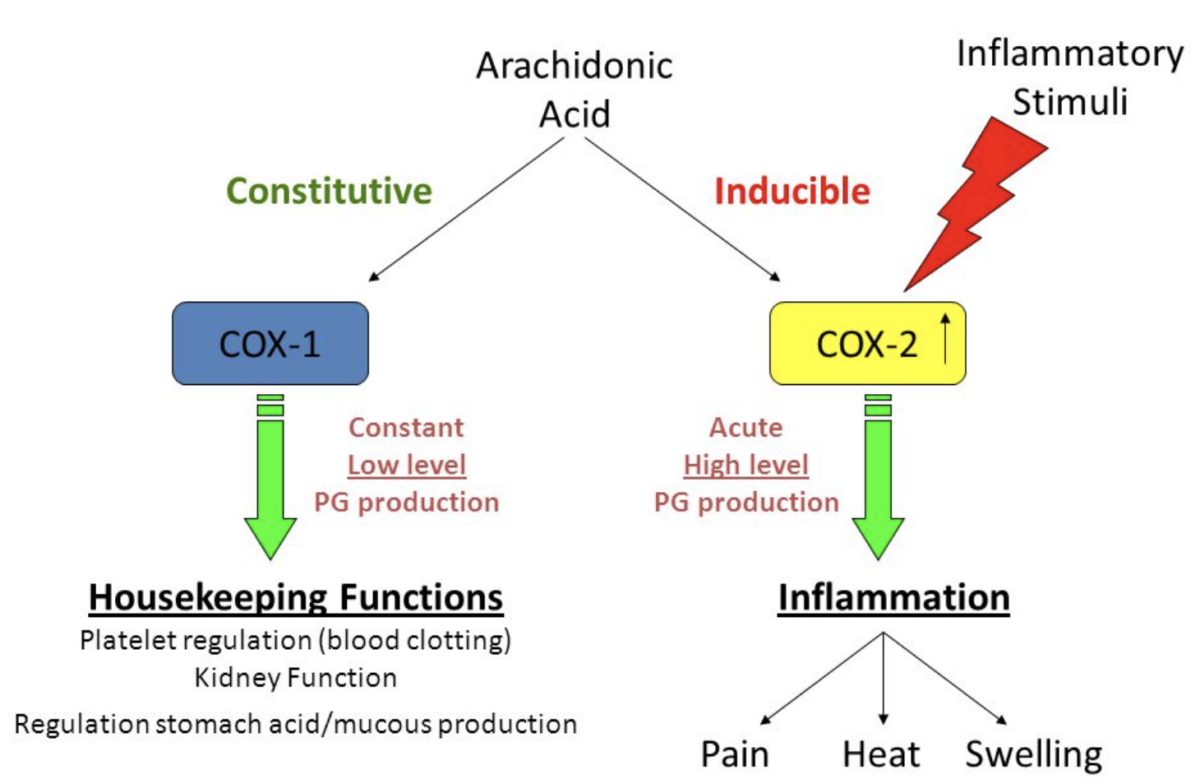
To know how NSAIDs work we have to understand the role of an enzyme called cyclooxygenase (COX). COX is responsible for the formation of thromboxane and prostaglandins such as prostacyclin from arachidonic acid (5).
NSAIDs work by inhibiting COX. Hence, they are often called COX inhibitors.
The inhibition of prostaglandin production reduces pain and inflammation. However, COX is also present in the gastric mucosa, where it stimulates gastroprotective prostaglandins, and thus helps to protect the innermost layer of the stomach.
There are two isoforms, COX-1 and COX-2 which play very different roles.
The pain-relieving and anti-inflammatory effects of NSAIDs are mediated through COX-2 inhibition, whereas the undesired effects on the stomach mucosa are linked to COX-1 inhibition.
The initial development of NSAIDs was not targeted at specifically inhibiting COX-1 or COX-2. Hence, frequently used NSAIDs such as aspirin, acetaminophen, indomethacin, ibuprofen, and naproxen, are regarded as non-selective COX inhibitors. These drugs, while reducing inflammation, also inhibit platelet aggregation (especially aspirin) and increase the risk of stomach ulcers and bleedings from the gastrointestinal tract.
Diclofenac and meloxicam are two non-selective cyclooxygenase inhibitors that show some selectivity toward COX-2. This probably contributed to the worldwide success of diclofenac as well as to the successful launch of meloxicam. Both of these compounds, however, still interfere with COX-1 activity at therapeutic doses.
COX-1 is found throughout most cell lines in almost all mammalian tissues. It is described as a housekeeping enzyme, being responsible for cell-to-cell signaling, tissue homeostasis, and cytoprotection. The term ‘cytoprotection’ means protection against injury to the mucosal line of the stomach by a mechanism other than inhibition or neutralization of gastric acid (6).
Hence, it is no surprise that NSAIDs, by inhibiting COX-1 make the stomach more susceptible to injury
Conversely, COX-2 is an inducible enzyme influenced by numerous chemical mediators that promote inflammation. Without appropriate stimulation, isolation of the COX-2 protein is negligible in most tissues.
COX-2 is considered to be a principal mediator of inflammation (7). Hence, in theory, inhibiting COX-2 but not COX-1 is targeted more specifically at the inflammatory process with less risk of gastrointestinal and other side effects.
Selective COX-2 Inhibitors (Coxibs)
Most NSAIDs, until the discovery of the COX-2 isoform in the early 1990s, were effective inhibitors of both forms of COX.
Soon after NSAIDs were introduced into clinical practice, it became clear that it might be advantageous to have drugs that only interfered with COX-2. The main reason was that this enzyme is not involved in the production of prostaglandins that protect the mucosal lining of the stomach.
A breakthrough came in the 1990s with the discovery of highly selective sulfonamides, celecoxib and valdecoxib, and the methylsulfones, rofecoxib, and etoricoxib. These compounds selectively inhibit COX-2.
Many experts believed that the introduction of selective COX-2 inhibitors would revolutionize anti-inflammatory drug therapy. However, these drugs are not without side effects, since inhibition of COX-2 affects kidney function, blood pressure, and possibly other physiologic parameters
In 2004, rofecoxib (Vioxx), marketed as a selective COX-2 inhibitor, was withdrawn from the market after the results of a randomized placebo-controlled trial showed an increased risk of cardiovascular events associated with the drug (7). This finding was confirmed in other trials and a cumulative meta-analysis (8)
Rofecoxib was withdrawn worldwide by the manufacturer in 2004 because of an increased risk of adverse cardiovascular events, and valdecoxib (Bextra) was withdrawn from the United States and European Union markets in 2005. Parecoxib, a water – soluble prodrug of valdecoxib, is administered intravenously and available in Europe but not in the United States.
Etoricoxib is another coxib that is available in Europe and some non-European countries, but it has never been available for use in the United States (9).
Currently, celecoxib (Celebrex, Celebra) is the only COX-2 inhibitor available in the United States. It is available as a generic.
The Adverse Effects of NSAIDs
More than 20 NSAIDs are available commercially. They are used worldwide for their ability to relieve pain, reduce inflammation, and decrease fever.
In general, NSAIDs are relatively safe drugs. However, there is a risk of adverse effects, particularly in patients at risk for gastrointestinal, renal, or cardiovascular events. Furthermore, NSAIDs can interact with many other drugs. Hence, a thorough medical history and evaluation of current drug use are of key importance before therapy is initiated.
Gastrointestinal Effects
NSAIDs can cause upper and lower gastrointestinal side effects ranging from mild irritation to more severe adverse events such as bleeding and perforation. This is particularly true for non-selective NSAIDs.
Upper intestinal side effects include indigestion, heartburn, nausea, and upper abdominal discomfort. Injury to the mucosal lining of the stomach and duodenum may result as well. When ulceration develops into bleeding, endoscopic therapy, and high dose proton pump inhibitor are recommended (11).
Lower gastrointestinal side effects, affecting the small or large intestine, are also common in patients taking NSAIDs (12). These include ulceration, bleeding, strictures, or obstruction.
Management of serious gastrointestinal side effects always includes stopping the medication (13). In severe circumstances, endoscopic interventions, surgery, and bowel segment resection are necessary.
Celecoxib, a selective COX-2 inhibitor, has been found to correlate with fewer gastrointestinal side effects, especially in combination with a proton pump inhibitor (such as omeprazole, esomeprazole, and rabeprazole) (12).
Although proton pump inhibitors help prevent and treat upper gastrointestinal side effects from NSAIDs, they have not been shown to decrease the risk of lower gastrointestinal side effects.
Those at risk of gastrointestinal side effects include patients who are older than 65 years, have a history of peptic ulcer disease, or are also taking glucocorticoids, antiplatelet agents (eg, aspirin, clopidogrel) or anticoagulants (eg, warfarin, heparin, direct thrombin inhibitors, and direct Xa inhibitors) (10).
Untreated Helicobacter Pylori infection may increase the risk of bleeding or perforation during long-term use of NSAIDs (14).
If treatment is deemed necessary in patients at risk for upper gastrointestinal side effects, preventative strategies should be put in place. These include the use of the lowest effective dose of NSAID, co-therapy with a gastroprotective drug (eg, proton pump inhibitors), or the use of a COX-2 selective agent such as celecoxib.
For patients with a significant risk of gastrointestinal bleeding who require NSAID therapy, the current recommendation is the lowest possible dose of celecoxib together with a proton pump inhibitor (13).
The best strategy to prevent lower gastrointestinal complications has yet to be defined.
Cardiovascular Effects
NSAIDs, except for low dose aspirin increase the risk of cardiovascular events, namely myocardial infarction (heart attack), cardiovascular-related mortality, and stroke. However, the overall risk is less than the risk of gastrointestinal side effects (11).
The risk is small in patients without cardiovascular disease and slightly greater in patients with pre-existing cardiovascular disease.
In 2015, the FDA strengthened its label warning regarding NSAIDs and the risk of heart attack and stroke.
The risk of myocardial infarction has been observed to be at its peak with just 7 days of NSAID use. Celecoxib, however, requires 30 days of continuous use. In one study, this risk remained sustained for 3 weeks and 3.5 months after discontinuation of non-selective NSAIDs and celecoxib, respectively (14).
A safety analysis of six randomized controlled trials showed that the risk appears to be non-significant with low dose celecoxib, 400 mg daily. Therefore, in a patient with high cardiovascular risk requiring NSAID therapy, the current recommendation is celecoxib 200 mg daily (11). If a patient has a low risk of gastrointestinal side effects risk, naproxen is acceptable, as well.
Diclofenac has been shown to have the highest cardiac risk (15).
In people with heart failure, naproxen, ibuprofen, rofecoxib, celecoxib, and diclofenac have been shown to increase mortality risk. Diclofenac seems to be associated with the highest risk and naproxen the lowest (16, 17)
Renal Effects
All NSAIDs can have adverse effects on the kidneys.
The risk is highest in patients with dehydration (hypovolemia), prior kidney disease, or hypotension.
Acute renal failure, chronic renal failure, acute interstitial nephritis, sodium and fluid retention, and hypertension may all result from the use of NSAIDs (11).
Joint use of angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), or diuretics together with NSAIDs increases the risk of acute renal failure.
NSAIDs can cause sodium retention leading to weight gain and edema. Hyperkalemia (high blood levels of potassium) can occur as well. Celecoxib and diclofenac have a higher risk of causing hyperkalemia (18).
Effects on Blood Pressure
All NSAIDs, in doses adequate to reduce inflammation and pain, can increase blood pressure. This is true for people with normal blood pressure and those with hypertension (19).
These effects may contribute to the increase in cardiovascular risk associated with these drugs.
Also, NSAID use may reduce the effect of all antihypertensive drugs except calcium channel blockers (20).
The blood pressure raising effect of NSAIDs probably involves inhibition of COX-2 in the kidneys. This reduces sodium excretion and increases intravascular volume which may lead to edema.
Hematologic Effects
NSAIDs may increase the risk of bleeding. This is particularly important in patients who are at increased risk for bleeding, such as those taking certain blood thinners or patients with liver failure.
NSAIDs cause platelet inhibition by inhibiting thromboxane A2 production.
Aspirin, for example, causes irreversible inhibition of COX, and therefore, the duration of platelet inhibition lasts until 7 to 10 days after the drug is stopped.
Because non-selective NSAIDs reversibly bind with COX-1, the antiplatelet effects of aspirin may be suppressed when NSAIDs are co-administered (21).
COX-2 selective inhibitors do not impede the antiplatelet effect of aspirin.
Cancer
Many studies suggest NSAIDs may have favorable effects on the risk of developing cancer. For example, continuous aspirin use may be effective in preventing colorectal cancer (22).
Decreased risk of prostate and breast cancer has also been described with NSAIDs. However, one study has suggested that the use of non-selective NSAIDs may be associated with an increased risk of renal cancer (23).
Allergy and Anaphylaxis
Allergic reactions to NSAIDs are common and may lead to anaphylaxis, a severe, potentially life-threatening allergic reaction.
The symptoms of anaphylaxis include skin reactions like urticaria and angioedema. Angioedema is characterized by swelling of the skin and tissue just under the skin or mucous membranes. The swelling may occur in the face, tongue, larynx, abdomen, or arms and legs.
Pruritus (itchy skin), fast or slow heartbeat, low blood pressure, nausea, vomiting, and headache may also occur.
The most commonly described hypersensitivity reaction to NSAIDs is exacerbated respiratory disease.
Alcohol Use
Alcohol intake is a risk factor for gastrointestinal bleeding. The risk is exacerbated in individuals taking NSAIDs (24).
What Is the Benefit of Cox-2 Inhibitors (Coxibs) in Terms of Adverse Effects?
Several toxicities associated with NSAIDs use occur less frequently or to a lesser degree with coxibs than with most nonselective NSAIDs. For example, there is reduced gastrointestinal toxicity, minimal effect on platelet function, and reduced bleeding risk. Besides, the coxibs appear to have little risk of precipitating bronchospasm in patients with aspirin-induced asthma (8).
In patients with and without cardiovascular disease, the use of coxibs is associated with an increased risk of adverse cardiovascular events, including death, myocardial infarction (heart attack), heart failure, and stroke. To minimize the risk, coxibs should be prescribed at the lowest effective dose for the shortest duration possible.
There is evidence indicating that the cardiovascular risk may very low with low dose celecoxib, 200 mg daily (11).
Coxibs can induce several different forms of kidney injury. Those at highest risk are patients with chronic kidney disease or volume depletion due to dehydration which may for example result from vomiting or diarrhea.
Medications including diuretics, angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), and calcineurin inhibitors (CNIs) can increase the risk of kidney injury form treatment with coxibs.
Coxibs may be preferred over other NSAIDs in patients at risk for gastrointestinal side effects, where there is risk of bronchospasm or when there increased bleeding risk.

NSAIDs and Drug Interaction
NSAIDs can interact with numerous drugs.
Low Dose Aspirin
Low dose aspirin is commonly used to reduce the risk of cardiovascular events in patients with heart disease (3).
The cardiovascular benefits of low dose-aspirin may be attenuated by treatment with non-selective NSAIDs such as naproxen and ibuprofen. Thus, regular NSAID use should be avoided in patients taking low-dose aspirin for cardiovascular protection (25).
Blood Pressure Medications (Antihypertensives)
Hypertension and chronic pain frequently coexist, particularly among elderly patients. Therefore, concomitant use of NSAIDs and antihypertensive drugs is common.
Many antihypertensive agents act through renal prostaglandins. Examples are angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), beta-blockers, and diuretics. The efficacy of these drugs may be reduced by concomitant treatment with NSAIDs.
Furthermore, the interaction between NSAIDs and antihypertensive agents may increase the risk of acute kidney injury (26).
Calcium channel blockers may be the only class of antihypertensive agents that is not influenced by concomitant treatment with NSAIDs.
Anticoagulants (Blood Thinners)
Anticoagulants (blood thinners), also termed anticoagulants, are frequently used for several medical conditions. Their use has increased dramatically during the last decade because of their efficacy in reducing the risk of stroke in patients with atrial fibrillation.
Interactions between NSAIDS and several types of anticoagulants have been reported.
Concomitant administration of NSAIDs with vitamin K antagonists such as warfarin poses an elevated risk of thromboembolism and bleeding.
The use of NSAIDs together with direct OACs, including the direct thrombin inhibitor dabigatran and factor X inhibitors rivaroxaban, apixaban, and edoxaban, is associated with increased risk of major bleeding, stroke, and hospitalization (27).
Selective Serotonin Reuptake Inhibitors (SSRIs)
SSRI drugs are frequently used for the treatment of anxiety and depression.
The use of these drugs in combination with NSAIDs is associated with an increased risk of gastrointestinal complications (25).
Methotrexate
Methotrexate is a chemotherapeutic drug that is often used for the treatment of psoriasis and rheumatoid arthritis.
Several NSAIDs, including ibuprofen and naproxen, have been found to reduce the clearance of methotrexate through the kidneys which could lead to toxicity (24).
Corticosteroids
The combined use of NSAIDs and corticosteroids may increase the potential for serious gastrointestinal adverse effects (24).
The risk may possibly be lower if COX-2 specific NSAIDs are used.
NSAIDs and COVID 19
There have been some concerns about possible negative effects of NSAIDs in the setting of COVID-19. This was based on anecdotal reports of a few young patients who received NSAIDs early in the course of infection and experienced severe disease.
However, there have been no clinical or population-based data that directly address the risk of NSAIDs in this setting. Given the absence of data, the European Medicines Agency (EMA), WHO, and the United States National Institutes of Health (NIH) COVID-19 Treatment Guidelines Panel do not recommend NSAIDs be avoided when clinically indicated in patients with CIVID-19 (28).
Most clinicians prefer acetaminophen/paracetamol to reduce fever in patients with COVOD-19 disease. If NSAIDs are used, the lowest possible dose should be given.
The Take-Home Message
NSAIDs provide an effective treatment for musculoskeletal pain and many other pain conditions. Chronic conditions, such as osteoarthritis, often require long-term treatment with these agents.
Hence, there is no surprise NSAIDs are amongst the most commonly used medications in the world.
Many NSAIDs are available as over-the-counter drugs.
Most patients who take therapeutic doses of these drugs for short durations tolerate them well. However, during long-term treatment and among certain risk groups there is a significant risk of adverse effects, some of which may be severe.
Hence, there is a consensus among experts that paracetamol (acetaminophen) should be the first-line analgesic agent due to its favorable side effect and safety profile, even though they are less effective in pain relief than NSAIDs.
However, besides being a less effective pain medication, paracetamol may not be as safe as believed, both from a gastrointestinal and cardiovascular perspective not to mention the well-known toxic effects on the liver (especially at doses higher than 3 g daily) (12).
Due to their over-the-counter availability, it is very important for the general public to be aware of the potential risks associated with treatment with NSAIDs.
Before therapy with NSAIDs is started, the real need for it should be carefully assessed and the gastrointestinal, cardiovascular, and renal risks should be carefully quantified.
Health care providers should be instrumental in educating patients about using the lowest effective dose of NSAIDs for the shortest duration possible.

Thank you Dr Sigurdsson for an excellent review !
Based on this reading, I can only imagine the damage done to the internal human flora in your gut. My advice is to avoid pharma as much as possible. Find the source of your pain and figure out a more natural way to alleviate it if possible.
So having read all that….there has to be an alternative, possibly natural remedy for people with high inflammation, such as those of us who deal with the daily inflammatory effects of arthritis in just about every joint!! Not fun to live with that pain level everyday, damn difficult! And diet surely has to have something to do with reducing inflammation level??! The arthritis is compounding on all aspects of life, you try to exercise and you suffer with more pain, headaches (arthritis in the neck). No exercise, not good! This is beyond frustrating!!
I had waited a while to post this. I decided to stop taking Meloxicam 7.5 mg which I was taking say, 2-3 times a week, occasionally 4 times. I have no seriously painful arthritis at 83 and was using this NSAID to relieve symptoms from either overdoing things or from doing things the wrong way. In this approximately 7 week period of ‘abstinence’ I don’t feel that I have suffered through it. So my comment/question first is to say that for non-debilitating arthritis, maybe a NSAID is unnecessary. The question I will ask Dr. Sigurdsson is there medical evidence that getting over minor ‘aches and pains’ may be better achieved with no NSAID in order to let the body do its own work of recovery?
I will follow up on my post of two months or so above. I continue not to take Meloxicam and feel for milder conditions (most unlike the arthritis described by others) that NSAIDs are not helpful for me. I have discussed this with my doctor here and we both concur. It seems that before discontinuing this NSAID, I was taking Meloxicam every time I felt discomfort.
Hello. I read your interesting and informative article. I learned a lot that I did not know before.
But I don’t quite agree with some of the comments.
I have been living with arthritis for 3 years. And these are not the best 3 years of my life. Only taking medication saves me at least a little from the nightmarish pain in the joints.
I cannot imagine if these drugs were not available. My life would be hell.
I agree that certain medications have a profound effect on gut health. But here we have a choice of either an absolutely healthy intestine or a normal life.
Hi
I completely agree with you.
NSAIDs improve the quality of life of millions of people around the world.
Therefore, it is so important to understand how they work and what to be aware of when they are used.