Estimated reading time: 13 minutes
It’s called Lipoprotein(a), or Lp(a) for short. And if you haven’t had it measured, you’re not alone—most people haven’t.
In fact, most of you may have never heard of it.
It’s not part of a standard lipid panel. It doesn’t show up in routine check-ups. And yet, one in five people carry levels so high they double or triple their risk of a heart attack or stroke—even if their cholesterol is “normal.”
This isn’t the first time I’ve written about Lp(a).
Back in 2021, I published an earlier version of this article to spotlight a neglected piece of the cardiovascular puzzle. But now, the landscape is shifting.
Trials are underway. Guidelines have evolved. And for the first time, therapies built specifically to lower Lp(a) are on the horizon.
Because Lp(a) is no minor footnote in cardiovascular risk.
It’s a genetically determined, cholesterol-rich particle with a nasty habit of promoting plaque buildup, clot formation, and aortic valve calcification. It doesn’t respond to statins. Diet won’t touch it. And for decades, there was nothing we could do about it.
Now, that’s changing.
This updated article explores what Lp(a) is, how it causes harm, who should get tested, and why we may need to rethink what we mean by “normal” cholesterol.
The Lipoprotein That Got Left Behind’
What Is Lipoprotein(a) – Why Is It So Dangerous?
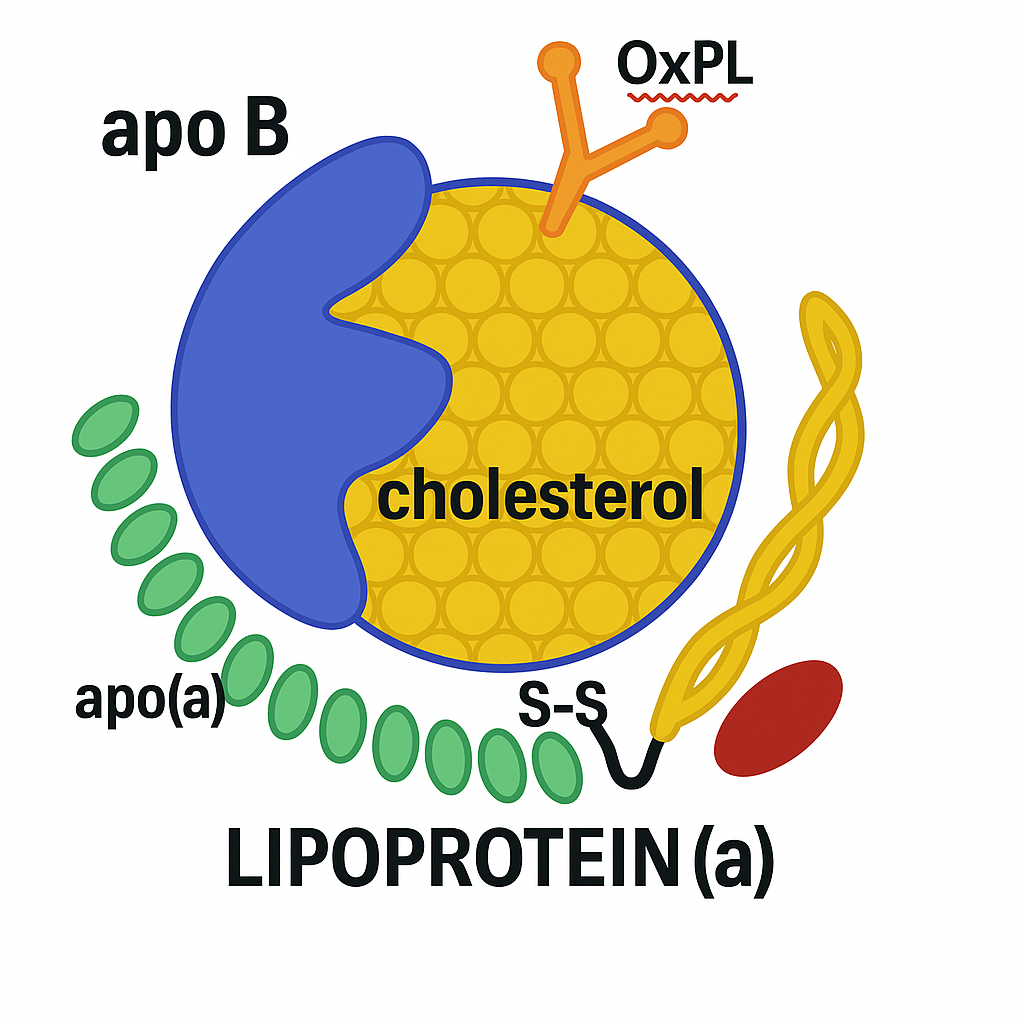
At first glance, Lipoprotein(a) looks like LDL.
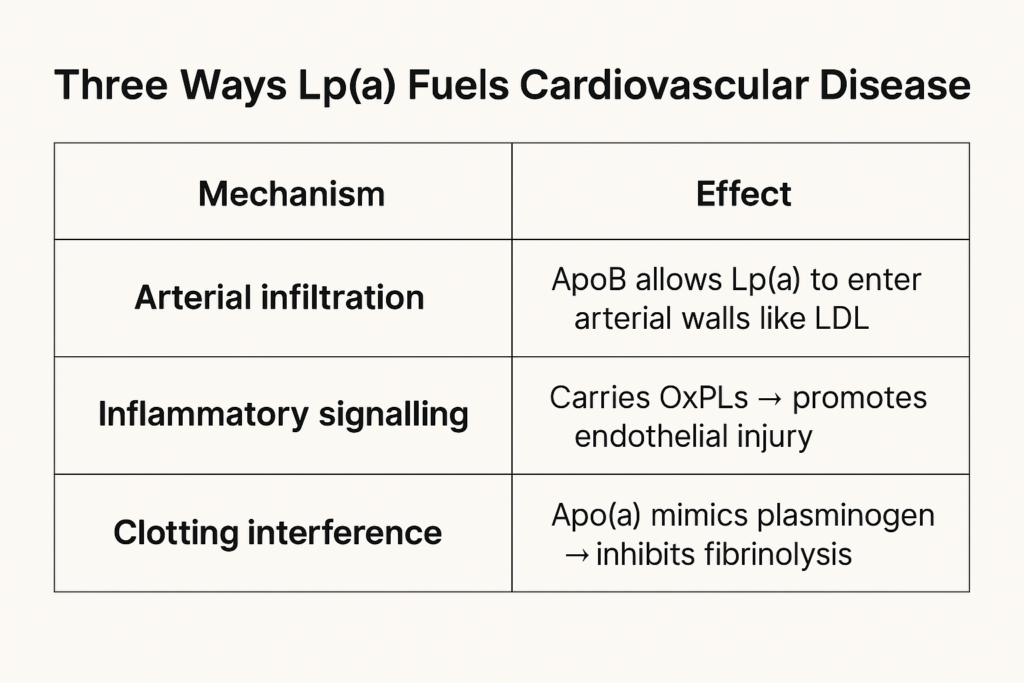
It carries cholesterol through the blood. It contains one molecule of apolipoprotein B100 (apoB). And like LDL, it’s atherogenic—capable of sneaking into artery walls and fueling plaque buildup.
But Lp(a) has a hidden weapon: a strange, sticky appendage called apolipoprotein(a)—apo(a) for short.
This tail-like structure is what makes Lp(a) different—and dangerous.
The Molecular Double Life
Apo(a) is structurally similar to plasminogen, a protein that helps break down blood clots. But unlike plasminogen, apo(a) can’t dissolve anything. In fact, it may compete with plasminogen for binding sites, inhibiting the body’s ability to clear clots.
That gives Lp(a) a double dose of danger:
- It promotes atherosclerosis like LDL.
- And it may enhance thrombosis like a clotting factor gone rogue.
But that’s not all.
Lp(a) is also a major carrier of oxidized phospholipids (OxPLs)—inflammatory molecules that accelerate endothelial dysfunction, arterial calcification, and plaque vulnerability.
Lp(a)’s unique biology also promotes valve calcification, particularly in the aortic valve—making it one of the few known lipoproteins directly implicated in both atherosclerosis and valvular heart disease.
LP(a) Is LDL – With a Hidden Load
Here’s something most lab reports don’t tell you:
Lp(a) cholesterol is included in your LDL-Cholesterol (LDL-C) measurement.
Because Lp(a) is, by definition, an LDL particle with an apo(a) tail, its cholesterol content is lumped into the LDL-C number reported on standard lipid panels.
That matters—especially in patients with high Lp(a). In fact, 10–30 mg/dL of your LDL-C may come from Lp(a) if your levels are elevated.
This has two major clinical implications:
-
After statin therapy, what’s left may be disproportionately Lp(a)-rich LDL-C—unchanged by the drug.
-
Residual cardiovascular risk may remain, even if LDL-C is “at goal.”
This is one reason why ApoB and non-HDL-cholesterol are more reliable risk markers than LDL-C alone—especially in patients with elevated Lp(a).
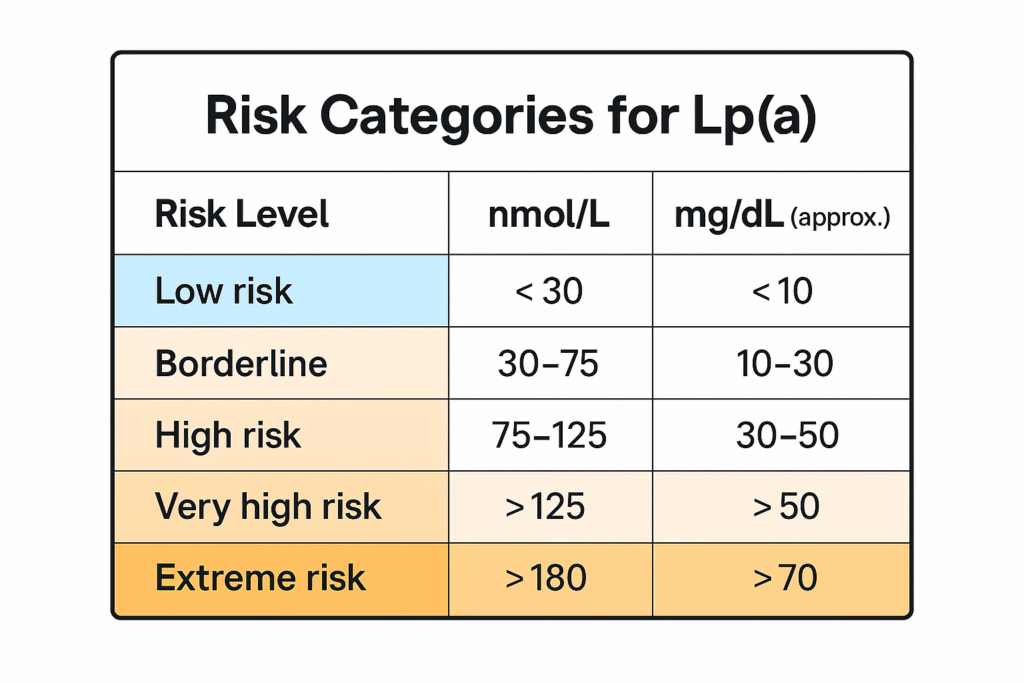
How Much Is Too Much? Lp(a) Levels and Cardiovascular Risk
One of the challenges with Lp(a) is that patients often have no idea what their number means—even if they’ve had it tested. The reference ranges vary by lab, and to make things more confusing, two different units are used:
-
nmol/L — particle concentration (preferred by most experts)
-
mg/dL — mass concentration
The same person might have an Lp(a) level of 120 nmol/L or 50 mg/dL, and both mean the same thing: high risk.
 What Patients Should Know
What Patients Should Know- There’s no clearly defined “safe” level of Lp(a)—but risk tends to rise as levels increase.
- Lp(a) is genetically determined. Lifestyle changes have minimal effect.
- It’s not part of a standard cholesterol panel. You have to ask for it.
- Most people only need to measure it once, unless their risk profile changes or new treatments become available.
When Lp(a) Testing Should Be Considered
- Personal history of early heart disease or stroke
- Strong family history of cardiovascular disease
- High calcium score or residual risk despite normal LDL
- Unexplained aortic valve stenosis
- Recurrent events on statins
Epilogue: The Ghost in the Blood
David was 52.
He didn’t smoke. He exercised. His LDL was borderline. Blood pressure, fine. HbA1c, normal.
So when he had a heart attack, it came as a shock to him and to his doctor.
They called it a fluke. Put in a stent. Started a statin. Life moved on.
Two years later, it happened again.
But this time, someone looked deeper. A lipid specialist ran a broader panel.
Lp(a): 245 nmol/L. Off the charts. Unchecked. Unmentioned. Unmeasured.
It wasn’t part of his standard lipid test.
It wasn’t flagged as a risk factor.
But it had been there all along—a silent, genetically programmed threat.
He didn’t look like someone on borrowed time.
He looked like every other man in the waiting room—shirt tucked in, Apple Watch on his wrist, a clean cholesterol printout in his hand. But under the surface, a ghost had been moving quietly for years.
That’s the reality for millions.
Not just the heart attack survivors, but the ones with unexplained strokes.
The ones with valve disease in their 50s.
The ones whose parents died too young.
This isn’t rare. It’s just rarely measured.
Now we have the tools to look—and soon, perhaps, the tools to treat.
Pelacarsen. Olpasiran. New names in a long-overdue war.
Not miracle cures. But a sign that medicine is catching up to what biology has always known:
Some people carry danger in their blood from the day they’re born.
And if we can find it early enough—we just might change the ending.
Related Reading on Doc’s Opinion
- Non-HDL Cholesterol: The Hidden Heart-Disease Predictor You’ve Overlooked (link)
- Atherogenic Dyslipidemia (link)
- When High LDL Leads to Heart Disease — And When It Doesn’t (link)
- The 12-Step Biology of Atherosclerosis (link)
- The Triglyceride/HDL Cholesterol Ratio (link)
- LDL-C vs. LDL-P: What’s the Difference? (link)
- What’s the Best Lipid Marker to Predict Risk? (link)
- Apolipoprotein B and Heart Disease (link)
- High Triglycerides — And How to Lower Them (link)
- VLDL, Triglycerides & Remnant Cholesterol (link)
- HDL Cholesterol — The Good, the Misunderstood (link)
- 10 Pitfalls of Using LDL-C to Assess Risk (link)
- The Role of Low-Density Lipoprotein (LDL) in Atherosclerosis and Heart Disease (link)
- LDL Particle Number vs. Size — Made Easy (link)
- LDL-P (link)
References
- Tsimikas S. A Test in Context: Lipoprotein(a): Diagnosis, Prognosis, Controversies, and Emerging Therapies. J Am Coll Cardiol. 2017;69(6):692–711. doi:10.1016/j.jacc.2016.11.042. Link
- Nordestgaard BG, Chapman MJ, Ray K, et al. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31(23):2844–53. doi:10.1093/eurheartj/ehq386. Link
- Boffa MB, Koschinsky ML. Lipoprotein(a): truly a direct pro-atherogenic particle. Curr Opin Lipidol. 2013;24(5):377–83. doi:10.1097/MOL.0b013e328363f6ab. Link
- Thanassoulis G, Campbell CY, Owens DS, et al. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368(6):503–12. doi:10.1056/NEJMoa1109034. Link
- Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, et al. Lipoprotein(a) Reduction in Persons with Cardiovascular Disease. N Engl J Med. 2020;382(3):244–55. doi:10.1056/NEJMoa1905239
→ (Pelacarsen Phase II trial). Link - Clarke R, Peden JF, Hopewell JC, et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361(26):2518–28. doi:10.1056/NEJMoa0902604. Link
- U.S. National Library of Medicine. HORIZON Trial (NCT04023552). ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ct2/show/NCT04023552
- U.S. National Library of Medicine. OCEAN(a)-Outcomes Trial (NCT05581303). ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ct2/show/NCT05581303
- Vinci P, Di Girolamo FG, Panizon E, Tosoni LM, Cerrato C, Pellicori F, Altamura N, Pirulli A, Zaccari M, Biasinutto C, Roni C, Fiotti N, Schincariol P, Mangogna A, Biolo G. Lipoprotein(a) as a Risk Factor for Cardiovascular Diseases: Pathophysiology and Treatment Perspectives. Int J Environ Res Public Health. 2023 Sep 6;20(18):6721. doi: 10.3390/ijerph20186721. PMID: 37754581; PMCID: PMC10531345. Link
- Khera AV, Everett BM, Caulfield MP, et al. Lipoprotein(a) concentrations, rosuvastatin therapy, and residual cardiovascular risk: an analysis from the JUPITER trial. Circulation. 2014;129(6):635–642. doi:10.1161/CIRCULATIONAHA.113.004406. Link
- Kamstrup PR, Tybjærg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301(22):2331–2339. doi:10.1001/jama.2009.801. Link
- Bittner V, Szarek M, Aylward PE, et al. Effect of alirocumab on lipoprotein(a) and cardiovascular risk after acute coronary syndrome. J Am Coll Cardiol. 2020;75(2):133–144. doi:10.1016/j.jacc.2019.10.057. Link
- Mehta A, Virani SS, Ayers CR, et al. Lipoprotein(a) and family history predict cardiovascular disease risk. J Am Coll Cardiol. 2020;76(7):781–793. doi:10.1016/j.jacc.2020.06.061. Link

How Lpa may enter the arterial wall?
Hi Gilles
It probably enters the vessel wall in a similar manner that LDL does: https://www.docsopinion.com/low-density-lipoprotein-atherosclerosis-heart-disease/
mine is <15, but I have a 362 CAC score, so it didn't help me any to have a low LP(a)
Hi Michael
I’m not sure I agree.
In theory, your coronary calcium score might have been higher than 362 if your Lp(a) was elevated.
And, of course, many other factors are involved in the calcification of the coronary arteries.
I’ve been getting my Lp(a) tested over the course of the last 8 years (went low carb/keto on 1/1/14), and my Lp(a) is ridiculously high over that time, between 220-360+ mmol/l, yet I got a zero score on CAC at about 5.5 year point. 90% of people had higher CAC scores (I was 55 at the time).
I also have no aortic stenosis, as verified by multiple transthoracic echocardiograms over that period.
Finally, I believe PUFAs cause higher values of Lp(a) and saturated fat can lower these values. I’m in the process of setting up a test of this to verify, though whether I can afford the costs of the multiple blood tests will be a factor as to whether I can do the actual test.
interesting, dr. sigurdsson, no lab report has included lp(a). everything else it seems is there!
Lp(a) is due because the human body cannot produce vitamin C. As a result Lp(a) is the patchwork necessary when micro tears occur within the arterial wall (i.e. endothelium aka glycocalyx). This is well documented by the research done by Drs. Rath & Pauling.
Dr. Rath concluded that sufficient amounts of L-Pro and L-Lys would in effect remove the Lp(a) residue. This in conjunction with mega-dosing Vit. C would far exceed niacin therapy or PCSK9 both of which only reduce 20-30 % Lp(a). B3 over time raises FBS, PCSK9s have been reported to cause ‘neurocognitive effects’ (attention deficit and confusion) and Antisense Oligonucleotides have an FDA Warning label for hepato-toxcicity, elevated serum transaminase, and hepatic steatosis
Thanks Doc! Particularly relevant to both myself and family. My father recently had a TAVI due to AO. A background in endurance sports (Int. level) and still riding at 82yrs with very clear coronary arteries. We have near identical cholesterol profiles and I’ve been logging miles most of my life. A recent echo showed no stenosis but it’s on my radar and won’t be unexpected. Keep writing!
Speaking of entering the arterial wall, a research paper from the Salk Institute a couple of months ago seems to show that the spike protein used by Covid to enter cells and released/created? by mRNA vaccines may cause vascular damage.
=========
Salk researchers and collaborators show how the protein damages cells, confirming COVID-19 as a primarily vascular disease
April 30, 2021
https://www.salk.edu/news-release/the-novel-coronavirus-spike-protein-plays-additional-key-role-in-illness/
===========
Do you have any thoughts on this aspect of Covid & related mRNA vaccines?
I just received my full lipid panel after my dad passed away from atherosclerosis. My total cholesterol is 214, HDL 62, LDL-C 135, non-HDL 152, LDL-P 1,474, Apolipoprotein B 105, Lipoprotein 206, trigylcerides 71. I’m only 33, do not have high blood pressure or diabetes, but am slightly overweight. I’m not sure if I should be concerned or not, but given the circumstances with my dad recently passing, I’m scared. Should I be on medication or is this something I can correct with lifestyle modifications?
Yes I would be concerned. While you are still young you can control your high cholesterol levels by eating healthy and exercising regularly. However if you are not able to significantly reduce these mentioned cholesterol levels in another 6 months then you should be on low dose statins ( prescribed cholesterol lowering drugs)
So if you have a score of 9 nmol / L (lab ref value <75) for Lp(a) and HDL of 58, Triglycerides of 62, Total of 186 and LDL of 114 and hs-CRP of .45, yet have a CAC score of 385 in the LAD and 0 in two other arteries and 50 in the remaining one. Particle number 1164 (down from 1450 7 years prior), small LDL-P 202 nmol/L (lab ref 20.5). 70 years old with type 1.5 diabetes (20+years)rheumatoid arthritis of 20+ years (in remission with Enbrel for past 8 years). HBA1c of 5.8.
Eat 20% carbs, 25% protein, 55% fat. Walk 5 miles/day at 4mph pace – semi-hilly terrain. Heart rate gets up to 135 at peak of walk. No difficulties with breathing or chest pain.
What do you make of the CAC score and what to do about it based on my numbers and info above? Docs all want me on statins. Have declined this option due to having too many friends with serious issues from them. I am trying to eat a low inflammatory diet with high doses of fish oil, 1-2 grams of Kyolic Aged Garlic Extract, CoQ10 (200mg/day), multi-Vit, Quercetin, Circumin, folate,Vit D 5000IU,400-600mg of chelated Magnesium, Collagen powder. Thinking about taking d-ribose and l-carnitine per Dr Stephan Sinatra theory of providing additional energy to the heart.
Thanks for any thoughts you might have!
Mark
56 year old Male, CAC 12.9, 12 of that in Right coronary. TC 209, LDL 138, Trig 122, but I have high bp, especially in AM upon waking, varies wildly, e.g. 160/101 ten minutes after waking, down to 127/78 one hour later. Trying to fix diet, not easy.
Some Centenarians have elevated levels of Lipoprotein (a) so it might be protective or a good thing as it’s in the genetics for a reason.
https://pubmed.ncbi.nlm.nih.gov/9543111/
https://pubmed.ncbi.nlm.nih.gov/9535215/
I have a calcium score of about 3500
Had a double bypass January due to severely calcified blocked coronary veins
And have lp(a) level of 839 mg/l
What is my prognosis and can I do something to improve this besides healthy food and daily sport/ training what I am already doing
I use ezetimibe because cannot use statins due to muscle/ boneproblems
Axel, I am writing a paper on Lp(a) and would like to use your diagram of the LDL and Lp(a) particles if that is possible.
Thanks for a good article!
Hi Allen
That’s fine as far as I´m concerned.
Good luck with your paper.
Axel
Hi, could I use you LDL/Lp(a) image for a presentation? In this case, a picture is worth a thousand words, and I feel like your image is doing a particularly good job.
Thank you
Interesting and helpful. I’m not sure it is truly genetic and should only be tested once. Seems like there are factors at play that can modify score. Mine was 80 a few years back, and now it is around 115, while on combination treatment (crestor + zetia). Looking forward to the trial results, doesn’t sound like there is much else to do in the meantime :).