Two decades ago, many experts predicted that the modification of risk factors, in particular, the treatment of high blood pressure and lipid disorders, would eliminate coronary artery disease in 10 – 20 years. Unfortunately, this prediction was wrong.
Although mortality from cardiovascular disease has decreased in many countries, coronary heart disease remains a significant cause of death and disability worldwide. Furthermore, the increased incidence of obesity and type 2 diabetes may ultimately reverse the declining mortality trend from heart disease.
Although there has been some improvement, one has to wonder why we haven’t had more success in reducing the prevalence and mortality from heart disease? Some will claim it’s because we haven’t succeeded in reducing the influence of traditional risk factors, such as high blood cholesterol. They will say that cholesterol lowering drugs (statins) are still underused among individuals at high risk and that many patients with heart disease are still not treated to target levels of low-density lipoprotein (LDL) cholesterol. And they could be right. There are ongoing trials, among them the FOURIER trial, testing the hypothesis that further lowering of LDL cholesterol with PCSK9 inhibitors on top of statin therapy will improve prognosis among patients with cardiovascular disease.
Another reason for the limited success is the possibility that there is a missing link. This link may be inflammation. It has been suggested that inflammation may play a major role in cardiovascular disease. If so, how can inflammation be modified? To be able to answer this question we will have to start with the basics. What is inflammation? How is inflammation involved in heart disease? Will reducing inflammation lower the risk of heart disease?
When we talk about heart disease in adults, we usually mean atherosclerotic coronary artery disease. This disease was first described in the eighteenth century. However, its most serious clinical entity, acute myocardial infarction, generally known as acute heart attack was not recognized until the early twentieth century.
In the 1950’s acute myocardial infarction was recognized as one of the most common causes of death in the industrialized world. The symptoms were often dramatic and devastating. A previously healthy person was suddenly hit by severe chest pain, often associated with serious disturbances in heart rhythm, frequently resulting in sudden death. The survivors often had to deal with the consequences of damage to large parts of the heart muscle, sometimes resulting in heart failure, severely compromised quality of life and a shortened lifespan.
Acute myocardial infarction occurs when there is a sudden disruption of blood flow in a coronary artery. The coronary arteries supply blood to the heart muscle. A sudden blockage is usually caused by a rupture of an atherosclerotic plaque within the vessel wall, with subsequent formation of a blood clot (thrombosis) at the rupture site. A sudden disruption of blood flow causes the death of heart muscle cells (infarction) and may impair the function of the heart muscle.
The hunt for conditions that predispose to acute myocardial infarction was well on its way by the mid-1950’s. In 1961 the Framingham team reported that high blood levels of cholesterol and high blood pressure were associated with increased risk of coronary artery disease. The term “coronary risk factors” was defined, and researchers were able to gradually uncover other conditions which predispose to this disease, such as cigarette smoking, the various fractions of cholesterol, insulin resistance, physical inactivity, mental stress, depression and dietary factors. However, although many risk factors have been identified and modified by preventive measures, coronary artery disease remains a common disorder. Despite extensive research, our understanding of the mechanisms behind coronary artery disease and acute clotting of diseased arteries is incomplete.
Today most scientists believe that inflammation plays a key role in atherosclerosis and acute myocardial infarction. As a matter of fact, signs of inflammation at the sites of atherosclerotic plaques have been observed for centuries. In the nineteenth century, there was a fierce controversy between the prominent Austrian pathologist Carl von Rokitansky and his German counterpart, Rudolf Virchow. While the former attributed a secondary role to these inflammatory arterial changes, Virchow considered them to be of primary importance.
Today, almost two centuries later, important issues remain unresolved. How can vascular inflammation be measured and quantified? Which inflammatory mechanisms are most important when it comes to atherosclerosis and coronary artery disease. How can vascular inflammation be reduced or modified? Will measures that reduce inflammation affect the risk of atherosclerotic heart disease and its consequences?
What Is Inflammation?
Inflammation is a protective tissue response to injury or destruction of tissues, which serves to destroy, dilute, or wall off both the injurious agent and the injured tissues. The classical signs of acute inflammation are pain (dolor), heat (calor), redness (rubor), swelling (tumor), and loss of function (functio laesa).
A good example of inflammation is when we get a splinter in our finger. The redness is caused by increased blood flow. The swelling is partly caused by white blood cells dispatched by the immune system to destroy the attacker and repair the injury. So, obviously, inflammation is one of the body’s most important defense mechanism. Without it, we would not be able to fight bacterial infections, injuries, and destruction of tissues. So, how can inflammation be harmful?
The body’s defenses are controlled by the immune system. The immune system is composed of biological structures and mechanisms that continuously protects us against diseases such as infections and cancer. Immune deficiency is associated with increased risk for these diseases. Autoimmune disorders such as rheumatoid arthritis, Hashimoto’s thyroiditis, systemic lupus erythematosus and type 1 diabetes are all associated with a dysfunction of the immune system.
Inflammation can be both acute and chronic. Acute inflammation is the initial response of the body to harmful stimuli. Prolonged inflammation or chronic inflammation is characterized by simultaneous destruction and repair of the tissue from the inflammatory process.
When inflammation is appropriate, it protects us from disease. When inflammation is inappropriate or gets out of hand, it can cause disease. Autoimmune disorders are characterized by an inappropriate immune response against cells and tissues in our body. This commonly leads to inflammation of tissues and organs such as joints, endocrine organs like the pancreas and thyroid gland, visceral membranes and internal organs such as the lungs, kidneys and blood vessels. Vasculitis is a term that is commonly applied to autoimmune inflammation of arteries.
Inflammation and Heart Disease
The wall of most human arteries is composed of three layers. The innermost layer is the endothelium which overlies an intima of extracellular matrix and smooth muscle cells. The next layer, the media contains mainly smooth muscle cells and extracellular matrix. The outermost layer, the adventitia, consists of looser connective tissue, nerve endings, mast cells and the so-called vasa vasorum.
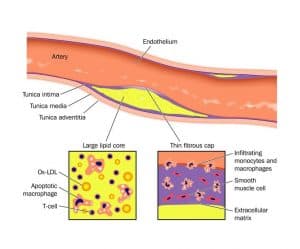
Studies have indicated that the infiltration and retention of low-density lipoprotein (LDL) in the arterial intima initiate an inflammatory response in the artery wall. Modification of LDL, through oxidation or enzymatic attack in the intima, causes the release of phospholipids that can activate endothelial cells. Studies in animals and humans also indicate that high blood levels of cholesterol may cause focal activation of vascular endothelium.
Cholesterol crystals are needle like structures that are found in atherosclerotic plaques. The role of these crystals in the atherosclerotic process is unknown. It has been proposed that cholesterol crystals may play a central role in initiating inflammation in atherosclerosis.
Recruitment of white blood cells (leukocytes) to the arterial wall is an early event in the formation of an atherosclerotic plaque. What triggers leukocytes to adhere to the vascular wall is unknown. A widely accepted view suggests that prolonged high levels of LDL particles in the blood stream may promote an infiltration of these particles to the arterial intima. Indeed, experimental animals begin to recruit inflammatory leucocytes soon after starting a diet enriched in cholesterol and saturated fat.
When inside the vessel wall, some leucocytes (monocytes) change into so-called macrophages. These cells are prominent in the atherosclerotic plaque. Macrophages may ultimately be transformed into foam cells, the prototypical cell in atherosclerosis. The activated macrophages produce inflammatory cytokines and other substances. Many other types of leucocytes are found in atherosclerotic plaques which underline the important role of the immune system and inflammation in the formation of atherosclerosis.
The ultimate complication of atherosclerosis, the formation of the blood clot or thrombosis, also appears to depend on inflammation. A disruption or rupture of an atherosclerotic plaque is the process that most often triggers thrombosis. The most common form of plaque disruption, rupture of the plaque´s protective fibrous cap, relates closely to inflammatory processes. Plaques that tend to cause fatal coronary thrombi often contain large accumulations of inflammatory cells. They also typically have a thin protective fibrous cap that overlies the lipid core.
Interestingly, many thrombotic occlusions of a coronary artery, resulting in myocardial infarction, do not occur at the sites of critical narrowing of the artery. Rather, lesions that do not cause critical stenosis often underlie clots that cause acute myocardial infarction.
The balance between inflammatory and anti-inflammatory activity controls the progression of atherosclerosis and thrombosis. Metabolic factors also affect this process. Lipid deposition in the artery may initiate inflammation.The adipose tissue of patients with the metabolic syndrome and obesity produces inflammatory cytokines that may promote vascular inflammation.
Inflammatory Biomarkers
A biomarker is a substance that can be measured, usually in blood, and reflects a biological state. Biomarkers reflecting inflammation can help identifying and quantifying inflammation.
C-reactive protein (CRP) is a biomarker of low-grade inflammation.
Despite a lack of specificity for the cause of inflammation, data from a number of epidemiologic studies have shown a significant association between elevated serum plasma concentration of CRP and the prevalence of underlying atherosclerosis, the risk of recurrent cardiovascular events among patients with established disease, and the incidence of first cardiovascular events among individuals at risk for atherosclerosis.
Also, some drugs used in the treatment of heart disease, such as aspirin and statins, reduce serum levels of CRP. Reduced inflammation may contribute to the beneficial effects of these drugs.
CRP can be measured using various assays with different testing characteristics. The high sensitivity CRP assay (hs-CRP) is the most used assay to determine cardiovascular risk.
Lipoprotein-associated phospholipase A2 (LP-PLA2) is an emerging inflammatory marker. It is a lipoprotein-associated enzyme secreted by macrophages. Elevated Lp-PLA2 has been shown to predict the risk of myocardial infarction and stroke in population studies.
Other examples of inflammatory biomarkers are Interleukin-6 (IL-6) and fibrinogen.
Diet and Inflammation
The role of chronic inflammation in heart disease and other chronic diseases has stimulated research into the effects of diet, nutrition and other lifestyle measures on inflammatory markers. Although this research is still in its infancy, some knowledge is available on the relationship between dietary patterns and systemic inflammation.

Consumption of trans fats has been associated with markers of systemic inflammation.
Intake of omega-3 fatty acids has been associated with low levels of IL-6, suggesting an anti-inflammatory effect of omega-3.
Consumption of omega 6 fatty acids shows variable effects on inflammation. Both pro-inflammatory and anti-inflammatory effects have been described.
It has been suggested that high levels of dietary omega-6 may increase the amount of omega-3 needed to reduce inflammation. Consumption of carotenoids, flavonoids, and magnesium has been associated with lower levels of inflammatory markers.
Numerous studies have shown an association between fruit and vegetable consumption and low levels if inflammatory markers.
Much of the research on dietary patterns and inflammation has looked at the Mediterranean diet or its components. Adherence to a traditional Mediterranean diet has been associated with a 9% reduction in total and cardiovascular mortality, 6% reduction in cancer, 13% reduction in Parkinson’s and Alzheimer’s disease incidence. All these diseases have been associated with low-grade systemic inflammation.
High intake of olive oil, vegetables. Legumes, fruits, and fish have been associated with low levels of hs-CRP, suggesting that these foods may reduce inflammation. In the ATTICA study adherence to the Mediterranean diet was associated with lower levels of hs-CRP.
Will Reducing Inflammation Help?
Whether inhibition of inflammation will prevent heart disease, or improve prognosis in those with known disease, is currently a major unresolved issue in clinical care. Much of the data evaluating the impact of atherosclerotic therapies on inflammatory biomarkers and clinical events has derived from aspirin or statins, agents that not only reduce inflammation but that either inhibit platelet function (aspirin) or significantly lower LDL cholesterol (statins)
The JUPITER trial demonstrated that potent statin therapy reduces the risk of heart attack and stroke among individuals with low levels of LDL-cholesterol who are at risk due to elevated levels of hs-CRP. It is not known whether the clinical benefits of treatment are due to LDL-reduction alone, to inflammation inhibition, or to a combination of both processes.
Two large clinical trials are underway to address the hypothesis that lowering inflammation will lower event rates and improve prognosis among patients with heart disease.
The CANTOS trial evaluated whether interleukin-1ß (IL-1ß) inhibition with the drug canakinumab can reduce the rates of myocardial infarction, stroke, and cardiovascular death among patients with a history of previous myocardial infarction and elevated levels of hsCRP (> 2 mg/L). It is a landmark study because it showed for the first time that blocking an important component of the inflammatory cascade involved in atherosclerotic heart disease is associated with an improved outcome.
The CIRT trial which is funded by the National Heart, Lung, and Blood Institute (NHLBI) will evaluate whether low dose treatment with methotrexate will reduce major vascular events among patients with a history of myocardial infarction and either diabetes or the metabolic syndrome. Methotrexate is commonly used in the treatment of autoimmune disorders such as rheumatoid arthritis and psoriasis arthritis.
Discover more from Doc's Opinion
Subscribe to get the latest posts sent to your email.


This is an excellent review Axel. I often wonder what role airpollution (which we know increases the risk of myocardial infarction) and all the man made chemicals we are exposed to play in causing inflammation.
Barbara Roberts, MD
Thanks Barbara. I just stumbled on this very recent article touching on the relationship between air pollution and atherosclerosis.
Vitamin D appears important in controlling the extent to which this occurs
“When inside the vessel wall, some leucocytes (monocytes) change into so-called macrophages. These cells are prominent in the atherosclerotic plaque. Macrophages may ultimately be transformed into foam cells,”
https://www.plosone.org/article/info:doi/10.1371/journal.pone.0054625
Thanks Raymund. That´s interesting. Thus, vitamin D deficiency could be one of the factors contributing to inflammation.
I think Dr. Bill Lands has a good grasp of inflammation. https://videocast.nih.gov/summary.asp?live=8108&bhcp=1 Dr. Lands begins speaking around minute 12.
At about minute 19 Dr. Lands says, “Did you know that peanuts contain 4,000 milligrams of omega-6 per 28 gram, one ounce serving of peanuts… and one milligram of omega-3? Oops!” When I heard this, I realized my mistake. I’d been consuming a peanut butter sandwich for lunch almost daily for decades. Two months after I stopped consuming peanut butter my leg pains subsided. I watched that lecture in November 2009. I have since regained considerable strength and stamina. I have also been researching the omega-6 hazard. At this juncture I’d fairly confident that excessive omega-6 intake constitutes a public health disaster of unimaginable proportions. Sadly, mainstream nutrition science authorities still say that saturated fats are the culprit.
Data is rapidly accumulating on diet and inflammation. See for example this open access review paper: https://goo.gl/HTuuQ
My toughts on fats and inflammation https://goo.gl/TlVtc
Excerpt from thisAJCN article: https://ajcn.nutrition.org/content/83/6/S1505.full
“Inflammation is an overt or covert component of numerous human conditions and diseases. Although the inflammation may afflict different body compartments, one common characteristic of these conditions and diseases is excessive or inappropriate production of inflammatory mediators, including eicosanoids and cytokines. The roles of n−6 and n−3 PUFAs in shaping and regulating inflammatory processes and responses suggest that the balance of these fatty acids might be important in determining the development and severity of inflammatory diseases. For example, a high intake of n−6 PUFAs, especially arachidonic acid, could contribute to inflammatory processes and so could predispose to or exacerbate inflammatory diseases. Conversely, the recognition that the long-chain n−3 PUFAs have antiinflammatory actions suggests that increasing their intake by patients with inflammatory diseases, for example, through dietary supplementation, may be of clinical benefit. Possible therapeutic targets for long-chain n−3 PUFAs are listed in Table 3⇓. Supplementation trials have been conducted for most of these diseases. Those trials dealing with rheumatoid arthritis, inflammatory bowel diseases (Crohn disease and ulcerative colitis), and asthma will be reviewed in some detail here. This is because a larger number of trials have been conducted for these diseases or because the evidence of benefit is strongest in these diseases.”
Typically, scientists assume that balancing n-6 and n-3 PUFAs is best achieved by increasing n-3 intake, not decreasing n-6 intake. Consequently, there are no long term clinical trials testing whether decreasing intake of n-6 would be of benefit.
Regarding saturated fats and inflammation, while it’s true that high serum levels of saturated fat induce an inflammatory response, high intake of saturated fats, in conjunction with low carbohydrate intake, reduces serum triglycerides. https://rdfeinman.wordpress.com/2012/02/22/saturated-fat-on-your-plate-or-in-your-blood/
Thanks for your comments David. I think there is a lot of evidence indicating that the omega-6:omega-3 issue is of importance when it comes to inflammation. Possibly, as suggested in your post, we should focus more on increasing omega-3 instead of reducing omega-6. There is lot of evidence indicating that increasing omega-3 has health benefits, but as pointed out in your post, there are no long term clinical trials that have tested whether reduced intake of omega-6 is beneficial.
By the way, I didn´t realize nuts contained so much omega-6.
Just like Reijo’s slides point out (and as he and I have earlier stated), there is no quality evidence whatsoever indicating that getting the amount of n-6 fatty acids that we currently get from our diet (that is, the average Western diet) is problematic. Inflammation seems to become a problem only when the intake is considerably larger. And n-6 fatty acids do have anti-inflammatory qualities, as well.
About nuts: yes, many nuts (like peanuts, hazelnuts) have n-3/n-6 ratios in the range of about 1:10. However, that isn’t the case with all nuts: in walnuts the ratio is about 1:4. And there is a growing amount of (I dare say) pretty solid evidence that nuts are BENEFICIAL in terms of cardiovascular health.
There’s no reason to fear nuts. On the contrary, there is every reason to “go nuts”, that is, to encourage people to include nuts into their everyday diet.
Mie says, “…there is no quality evidence whatsoever indicating that getting the amount of n-6 fatty acids that we currently get from our diet (that is, the average Western diet) is problematic.”
I would say there is no quality evidence suggesting that intake of n-6 fatty acids above one or two percent of total calories is safe. Here’s the latest indication that n-6 is problematic. Excerpt:
Metabolic syndrome is often accompanied by development of hepatic steatosis and less frequently by non-alcoholic fatty liver disease (NAFLD) leading to non-alcoholic steatohepatitis (NASH). Replacement of corn oil with medium chain triacylglycerols (MCT) in the diets of alcohol-fed rats has been shown to protect against steatosis and alcoholic liver injury. The current study was designed to determine if a similar beneficial effect of MCT occurs in a rat model of NAFLD. Groups of male rats were isocalorically overfed diets containing 10%, 35% or 70% total energy as corn oil or a 70% fat diet in which corn oil was replaced with increasing concentrations of saturated fat (18:82, beef tallow:MCT oil) from 20% to 65% for 21 days using total enteral nutrition (TEN). As dietary content of corn oil increased, hepatic steatosis and serum alanine amino transferases were elevated (P < 0.05). This was accompanied by greater expression of cytochrome P450 enzyme CYP2E1 (P < 0.05) and higher concentrations of polyunsaturated 18:2 and 20:4 fatty acids (FA) in the hepatic lipid fractions (P < 0.05). Keeping the total dietary fat at 70%, but increasing the proportion of MCT-enriched saturated fat resulted in a dose-dependent reduction in steatosis and necrosis without affecting CYP2E1 induction. There was no incorporation of C8–C10 FAs into liver lipids, but increasing the ratio of MCT to corn oil: reduced liver lipid 18:2 and 20:4 concentrations; reduced membrane susceptibility to radical attack; stimulated FA β- and ω-oxidation as a result of activation of peroxisomal proliferator activated receptor (PPAR)α, and appeared to increase mitochondrial respiration through complex III. These data suggest that replacing unsaturated fats like corn oil with MCT oil in the diet could be utilized as a potential treatment for NAFLD. https://ebm.rsmjournals.com/content/238/2/151.abstract
The CVD/ NAFLD connection:
NAFLD is one of the most common causes of chronic liver disease. Patients with NAFLD have an excess prevalence of CV events and typically have an increase frequency of risk factors already known to be directly related to atherosclerosis. As a consequence, it remains unclear if the presence of fatty liver should be regarded as an independent risk factor for CV disease. https://www.eurekalert.org/pub_releases/2013-04/eaft-n1r042513.php
Typically, scientists like to blame saturated fat for any problems related to inflammation because high levels of palmitic acid are associated with inflammation. However some of them have noted that high intake of omega-6 appears to be problematic as well. Abstract:
"Non-alcoholic fatty liver disease (NAFLD) ranges from steatosis and hepatic insulin resistance to non-alcoholic steatohepatitis (NASH), advanced fibrosis and cirrhosis. NAFLD is now considered as the hepatic manifestation of the metabolic syndrome, and both are triggered by mechanisms including inflammation, lipid overload and oxidative stress in adipose tissue and liver."
Note: saturated fats do not oxidise. https://eathropology.com/2012/03/16/kinky-stuff-about-fatty-acids/
Remainder of abstract:
"Despite accumulation of numerous data on NAFLD physiopathology, therapeutic modulation of the pathways involved appear insufficiently efficient or associated with serious adverse effects. The increased prevalence of NAFLD and metabolic syndrome during the last decades was associated with deep modifications of dietary habits, especially increased fat intakes. Recent literature provides clues of increased saturated (SFA) and n-6 polyunsaturated fatty acids (PUFA) as well as reduced n-3 PUFA in the diet of NAFLD and NASH patients. Indeed, strong data support the detrimental role of high SFA and n-6/n-3 ratio as well as low monounsaturated fatty acids (MUFA) and n-3 PUFA on metabolic parameters, which are ameliorated by administration of n-3 PUFA and MUFA. Despite governments and health associations having revised their recommendations for n-3 PUFA intakes upward during the last decade, those are still inferior to levels proved of therapeutic efficiency and are still not reached in the general population. This short review discusses these issues and provides consequent pragmatic suggestions for enhanced dietary measures for prevention of NAFLD and metabolic syndrome in the general population." https://www.ncbi.nlm.nih.gov/pubmed/21299150
It is well established that n-6 intake has increased while saturated fat intake has decreased. So, one wonders why scientists continue to blame saturated fats for all our woes. Kris Gunnars, a medical student and personal trainer who reads books and research studies on health and nutrition comments on the omega-6 increase:
"In the past century, consumption of these oils has increased at the expense of other healthy fats like butter. They were labelled as “heart-healthy” and the governments all around the world encouraged us to eat more of them." https://authoritynutrition.com/are-vegetable-and-seed-oils-bad/
And so we did.
“I would say there is no quality evidence suggesting that intake of n-6 fatty acids above one or two percent of total calories is safe. Here’s the latest indication that n-6 is problematic. Excerpt:”
Dave, the day you a) stop flooding & quote mining and b) address the studies concerning n-6 & inflammation I’ve referred to earlier, I’ll consider replying to you in more details. In the meanwhile, I’ll simply ignore your posts.
Reijo. I looked at your slides. Interesting. Do you think the issue of omega-6/omega-3 ratio is overrated or irrelevant? Most of us generally think that increasing omega-3 is beneficial. But, what about reducing omega-6. Is it unnecessary? Can it be harmful? Would love to hear your thoughts.
I think the ratio is overrated. The role of fats per se is overrated.
The current intake of n-6 fats is near to optimal level in many countries like Finland (about 4,5 % of energy). Think, in Sydney Diet Heart intake of n-6 FAs was about 15 % of energy (supplement material table 6). If we used n-6 oils like in Sydney Diet Heart or in Rose Corn Oil studies, there would be need to reduce n-6 intake. But we do not. We use canola oil, olive oil and modern margarines instead.
Blogosphere and many review papers cite falsely that current intake of n-6 fats is between 10-15 % of energy. Such statements are pure rubbish as the total intake of PUFAs is less than 10 % in armost all western countries. Obviously, intake of n-6 FAs cannot be higher than the intake of PUFAs.
The role refined carbs, extra virgin olive oil, saturated fat, polyphenols (certain spices, fruit, berries and veggies) is underrated in inflammation.
Bill lands seems to suggest that taking more omega 6 or more omega 3 will do very little because omega 6 is already too high. So the bad effects of adding extra Omega 6 and the beneficial effects of increasing omega 3 are already crowded out .
Charles Serhan is one of the go to guys for information on inflammatory response. Google: Charles Serhan Nutrition, Trauma, and the Brain PDF
Excerpt: “The prevailing theory on the actions of n-3 fatty acids (DHA, EPA) is that they compete for arachidonic acid in phospholipid stores, blocking the biosynthesis of eicosanoids that are proinflammatory such as leukotrienes and prostaglandins as well as vasoactive thromboxane. For an elegant review of this thesis, the readers are directed to Lands(2005). This competition between n-3 and n-6 pathways is evident with results from many studies and is demonstrable in vitro with isolated cells and enzymes with high concentrations of EFA (e.g. micro- to millimolar range) particularly DHA and EPA.”
Axel,
I think Daniel Steinberg (2008) covered the inflammation issue pretty thoroughly. Why we are not having more success is because people are being treated too late in life. The mean age at the randomization in large statin trials is 63. Even though cholesterol is reduced and plaque build-up is somewhat regressed, the more stable plaques can still cause a rupture.on people with existing CHD or several decades cumulative burden of elevated cholesterol.
Steinberg (2008) covers the very detailed mechanical models which indicates the inflammation is ceased once lipids are normalized. Moroever, he highlights the well established ecologic evidence:
“Taken together, all of these findings suggest that the inflammation associated with atherogenesis is not sufficient in itself to cause further lesion progression or even to maintain lesions at a steady state once the hypercholesterolemia has been fully corrected. In other words, many (or even most) of the inflammatory processes in the advancing lesion are downstream responses ultimately traceable to hyperlipidemia and its consequences. Consequently, early and aggressive correction of hypercholesterolemia may be sufficient. On the other hand, if hypolipidemic therapy is initiated at, say, 40 or 50 years of age, optimal intervention will no doubt also require attention to inflammation, thrombosis, and hemodynamic factors”
“One important line of evidence comes from a consideration of the Japanese experience. In 1952, mortality from CHD among Japanese men 55 to 64 years of age was <10% of what it was in the United States.15,16 Their total cholesterol levels at the time averaged ≈160 mg/dL (estimated LDL, ≈80 mg/dL). It is noteworthy that the Japanese enjoyed this relative immunity to CHD despite the fact that the prevalence of one of the major risk factors—cigarette smoking—was much higher in Japan than in Western countries,17 and another—-hypertension—was just as high.18 Even the diabetic population in Japan fares better than the diabetic population in Western countries. In 1985, almost 30% of British male diabetics but only ≈15% of the Japanese male diabetics had CHD.19 The implication is that if blood cholesterol levels are sufficiently low, the other dominant risk factors, including cigarette smoking, hypertension, and diabetes mellitus, constitute much less of a threat”.
A crucially important point needs to be noted here: For whatever reasons, the Japanese have their lower cholesterol levels for their entire lifetimes. Lowering the cholesterol level of a 50-year-old American for just 5 years, even if his cholesterol is successfully brought down to a Japanese-like level, is unlikely to convert his risk to a Japanese-like risk
https://circ.ahajournals.org/content/118/6/672.full
The legendary diet-heart specialist Jeremiah Stamler who did not let Siri-Tarino et al go too easy (Stamler 2010) is not too keen on the inflammation hypothesis either:
Causes and Mechanisms: An Interview With Jeremiah Stamler
“Is inflammation a component of atherogenesis? Of course. All you have got to do is look under the microscope. There are inflammatory cells and a fibrous reaction et cetera, et cetera, up to and including calcification and bone formation. This has all been well known for more than a hundred years. The human body has a limited set of ways for reacting to and attempting to combat adverse exposures, both infectious and noninfectious. Inflammation is a key reaction. So, inflammation is a part of the atherosclerotic process. However, from an etiologic point of view, is it central and critical? No way.:
https://journals.lww.com/epidem/Fulltext/2006/03000/Causes_and_Mechanisms__An_Interview_With_Jeremiah.21.aspx
Now Richard, care to explain what relevance does this “SOS” that you keep copy/pasting everywhere have concerning the blog post at hand?
Mie,
you should thank me, you cannot go wrong by much while copy/pasting Stamler and Steinberg.
Axel,
I come from a business background. We tend to study and scrutinize best-practise cases. I thought this was the natural frame-work and mind-set for all people. If you want to be good at something, you study the people who are already good at it. If you want to prevent CHD, look for the population who do not have CHD and study whether there is something to learn from them (Campbell et al 1998). Now, if you were as excited about potatoes, oatmeal, corn & stone-ground whole-wheat bread as you are about inflammation and high-fat diets, maybe you could actually one day prevent a CHD or two.
Richard,
I don´t come from a business background. However, I have performed and participated in scientific work for many years. I don´t believe we can accept or reject a hypothesis until it is tested. That is definitively true for the inflammatory hypothesis. I don´t believe, as you seem to do, that atherosclerosis and coronary artery disease are only about cholesterol. Although there is a significant relative reduction in clinical events in many of the statin trials, there is a number of events among those on statins as well. Many patients who aret treated to target levels of LDL cholesterol have acute coronary events. Remember also that the beneficial effects of statins may, at least partly be due to their anti-inflammatory effects.
I find your analogy with inflammation and potatoes a bit strange, maybe naive at best. There is a reason why the inflammatory hypothesis is currently being tested in large scale clinical trials. Potatoes, oatmeal, corn & stone-ground whole-wheat bread are not.
One of my main objectives when I write a blog article of this kind is to be informative. I´m not a politician. I´m not promoting a certain view. Of course we are all entitled to an opinion. I know that you have one and I respect it.
Dear Axel,
I am certain that we are seeing success while treating inflammation also in terms of clinical end-points. And, certainly these issues must be raised also for the lay-people. However, I am still not sure whether the newly found focus on inflammation will be serving the big audience. I think Jeremiah Stamler had some good points in the interview.
I agree that one must pay attention to issue beyond cholesterol. This is more crucial when treating high-risk patients at old age. However, If people understood the importance of LDL cholesterol (including its causal role in influencing CAD) and actually started taking some measures already way before mid-life, this additional focus would be rendered more irrelevant. To give you practical perspective, in the 7CS, 70% of participants smoked in the both Japanese cohorts at the baseline. Out of the smokers 40% smoked 20 fags or more. Blood-pressures were as high as in the West. Nevertheless, CHD is nearly non-existent in Japan at the time.
“I am certain that we are seeing success while treating inflammation also in terms of clinical end-points… However, I am still not sure whether the newly found focus on inflammation will be serving the big audience.”
This is besides the point. The focus here is that of an ADDED benefit. No one’s arguing – well, no one who’s to be taken seriously – that well-known lifestyle factors should be ignored.
Next time, put more focus on the issue at hand and less on the ecological fallacies such as leaving out the majority of the findings of 7CS.
Mie,
you are absolutely right. Axel had a very informative article, and I enjoyed updating myself about the on-going trials. My only “beef” with the article was the overall tinge. I think the readers ought to be informed about overall context. Inflammation is not a crucial phenomenon in the etiology of coronary artery disease. There are plenty of people who indeed think we can neglect the well-established evidence about the traditional risk-factors and just focus on inflammation.
“If you want to prevent CHD, look for the population who do not have CHD and study whether there is something to learn from them.”
That’s a good idea. The Kitava study is about one such population. https://www.staffanlindeberg.com/TheKitavaStudy.html
Interestingly, Kitivans consumed a low-fat/high-carbohydrate diet and about 77% of adults were daily smokers. Average cholesterol levels were higher in Kitiva than in Sweden. https://www.carbohydratescankill.com/2435/pearl-of-kitava-study-2-of-2
Comment:
“Although Kitavans took in less fat than Swedish from their respective daily energy intake, according to the Kitava Study, nearly 81% of the fat, which Kitavans consumed, was saturated; and slightly over 47% of the saturated fat consisted of middle-chain fatty acids. Interestingly, Kitavans used very little monounsaturated and polyunsaturated fats, which Swedish and other westerners were promoted as good source of fats. Because Kitavans depended on seafood very much and used nuts, they had a higher ratio of omega-3/omega-6 fatty acids than did Swedish and other westerners.”
I bet the Kitavans also had low levels of tissue HUFA, highly unsaturated fatty acids.
An important piece of the heart disease puzzle?
Newish study on omega 3 , Showing benefit
https://www.ncbi.nlm.nih.gov/pubmed/23546563
And on the Kitavans , the most significant difference between them and the Swedes
were insulin levels that were 50% lower .
“For example, the mean insulin concentration in 50- to 74-year-old Kitavans was only 50% of that in Swedish subjects. Furthermore, serum insulin decreased with age in Kitava, while it increased in Sweden in subjects over 50 years of age. ”
https://www.ncbi.nlm.nih.gov/pubmed/10535381
also some more on Vitamin D. I would guess the Kitavans have much higher vitamin D levels and stable levels too )
https://www.thisisreallyinteresting.com/research-shows-why-low-vitamin-d-raises-heart-disease-risks-diabetics/
A new RCT on fish-oil
https://www.nejm.org/doi/full/10.1056/NEJMoa1205409
(more discussion here: https://www.forbes.com/sites/larryhusten/2013/05/08/another-disappointing-study-for-fish-oil-supplements/)
showed overall null results for fish-oil supplementation in terms of primary endpoints (CVD death, nonfatal stroke & MI: later, admission to the hospital for CV causes was added) and secondary endpoints. However, women benefited from fish-oil supplementation.
Mie, Thanks for the info on fish oil. The data supports my contention that the absolute amount of polyunsaturated fatty acid intake becomes problematic in when it exceeds 2 to 3 percent of total caloric intake. Here’s further comment on the matter:
“Previous studies of human populations that consume large amounts of omega-3 fatty acids as part of their normal diet suggest a protective effect against cardiac and inflammatory disease.
But when researchers added the omega-3 rich fish oil to the diet of mice to see if it would reduce the inflammation caused by omega-6 rich vegetable oils, they were stunned when it made matters worse.
“Our hypothesis is that levels of omega 6 are so high in our bodies that any more unsaturated fatty acid — even omega 3, despite its health benefits — will actually contribute to the negative effects omega 6 PUFA have on the heart and gut,” said Ghosh. “When there is too much [polyunsaturated fatty acid], the body doesn’t know what to do with it.” https://life.nationalpost.com/2013/01/23/excessive-omega-fatty-acids-may-make-heart-health-worse-not-better-b-c-researchers/
Dave:
“The data supports my contention that the absolute amount of polyunsaturated fatty acid intake becomes problematic in when it exceeds 2 to 3 percent of total caloric intake.”
I’ll give it a try…
No Dave, it doesn’t. If you had cared to read any of the meta-analyses provided by me or Reijo, you’d noticed that there’s no proof of this.
And what comes to your mouse study, what relevance does it have in the context of the abovementioned results from human RCTs? Nada.
I don’t think that industrial fare (seed oils and margarine) sold to us as food has past the test of time. Anyone just looking for a reason to choose a prudent diet approach should at least read Chris Masterjhon take on the subject. I will keep avoiding them and not counting calories from anything, including omega-6 (mostly olive oil and nuts in my case).
Dave,
The Kitava folk make up a nice example of healthy & trim starch-based cultures indeed. However, they consume way too much coconut fat and hence show elevated cholesterol levels, although they seem to be protected from CHD. Anyway, there’s too much data against SFAs which cannot be overlooked due to this seemingly anomalous example. I would be very cautious with the use of saturated fat laden tropical oils. The molecular basis for the effects of dietary saturated fat on plasma LDL cholesterol levels is well understood. Saturated fat influences the LDL receptor activity of liver cells as described by Brown and Goldstein, dietary saturated fat suppresses messanger RNA synthesis for the LDL receptor resulting in increased LDL cholesterol concentration. Coconut oil is often used in experimental models to induce atherosclerosis.
1) Differences in all-cause, cardiovascular and cancer mortality between Hong Kong and Singapore: role of nutrition.
“There are striking differences in all-cause and cardiovascular mortality between Hong Kong and Singapore. These differences can be most reasonably and plausibly explained by their differences in dietary habits, for example, a higher consumption of coconut and palm oil, mainly containing saturated fat, in Singapore”
https://www.ncbi.nlm.nih.gov/pubmed/11855581
2) The emergence of coronary heart disease in populations of Chinese descent.
Dietary differences in saturated fat consumption were consistent with this. Although there was little difference in total fat intake, a higher consumption of dietary saturated fat and lower consumption of polyunsaturated fat, accompanied by higher serum cholesterol, appear to explain the relatively high CHD mortality in Singapore compared with Hong Kong and mainland China. Differences in body mass index, blood pressure and smoking between locations did not explain the differences in CHD mortality
https://www.ncbi.nlm.nih.gov/pubmed/12818413
3) Dietary Fat Intake and Carotid Artery Wall Thickness: The Atherosclerosis Risk in Communities (ARIC) Study
“After adjustment for age and energy intake, animal fat, saturated fat, monounsaturated fat, cholesterol, and Keys’ score were positively related to wall thickness, while vegetable fat and polyunsaturated fat were inversely related to wall thickness. These associations persisted after further adjustment for smoking and hypertension and were consistent across the four race and sex groups. Thus, elements of habitual dietary intake were consistently associated with carotid artery wall thickness, compatible with their putatively atherogenic and antiatherogenic properties”
https://aje.oxfordjournals.org/content/139/10/979.short
So, in Singapore, coconut oil causes CHD but in Kitava it doesn’t? To me that make no sense. What does make sense is that tissue levels of omega-6 HUFA predict CHD mortality. https://wholehealthsource.blogspot.com/2009/05/eicosanoids-and-ischemic-heart-disease.html
Note also that when total cholesterol is graphed against mortality, the safest cholesterol levels fall in the 200 to 240 range. https://perfecthealthdiet.com/wp/wp-content/uploads/2011/06/O-Primitivo-Cholesterol.jpg
40% of the Japanese smokers in the both cohorts smoked 20 cigarettes per day, and CHD was nearly non-existent in Japan at the time, that is. Moreover, I did not mean that potatoes and corn should replace pharmacologic interventions. What I meant was that these naturally low-fat food groups do wonderful things to LDL cholesterol and hence the emphasis should be on these foods in order to prevent CHD. After all, CHD is a food-borne disease.
“Dubbing this disease inflammatory obfuscates the fact that atherosclerosis is a nutritional-metabolic disease”.
–Jeremiah Stamler
It is stunning that Richard keeps pasting always ecological evidence. I think it is generally accepted that prospective cohorts and randomized clinical trials should be the kind of evidence with most value.
So why don’t we use them rather then the old ecological studies ?
And what do they tell us ? Prospective cohort studies suggest that there is no strong relation with amount of saturated fat intake and CHD. What does RCT tell us, it suggests that at best substituting saturated fat to unsatured fat may give modest benefits if any. Both study types have their problems and weaknesses as well. Even Darious Mozzafarian whose meta-analysis of RCT suggested some link between saturated fat and CHD thinks that we should not focus too much on saturated fat or to just one bio marker.
And lastly I think we should focus more on actual foods instead of some specific nutrients. We have quite strong evidence suggesting that it matters whether you take your saturated fat calories from meat or for example from dairy products.
^The issue you were discussing, ecologic evidence vs. prospective cohorts were recently discussed in the the two editorial articles of Europe’s leading diet-heart experts (13 of them). They were not too welcoming to the idea’s expressed by Mozzafarian and Co. I personally think that the idea’s held Mozzafarian are ill founded, and they ignore several lines of evidence (see Stamler 2010). But I am not a scientist, I want to only expose people to the “second opinion” which I think receives fairly little attention. Mozzafarian is the hero to those who want to downplay the importance of SFA reduction. However, Mozzafarian & Co has less influence over the recommendations of large scientific bodies. For example, the AHA recently opted even stricter upper-limits for SFAs. Moreover, in case you did not notice, I actually copypasted a study which measured dietary fat consumption with carotid wall thickness. Furthermore, online dietary guru’s who do not understand anything about statistical models or diet-heart research have spent hefty amount of time to brainwash laypeople into believing ecologic evidence is useless. Don’t expect to hear anything from regression dilution bias from these people.
The case against SFA would win in the court anytime, anywhere. We cannot overlook the darwinian foundation of our current biomedical research paradigm.
I wonder how long it takes before the advocates of “the big picture” to realize that their HDL-C sales-pitch ain’t goin’ nowhere anymore. It’s a single biomarker that is able to initiate atherosclerosis in every single model as well as in humans. It’s also the very lone and one biomarker that is causally related to CHD out of the many biomarkers in the lipid fraction (see the large Mendelian randomization analysis). I’m referring to LDL-C cholesterol.
The importance of reducing SFA to limit CHD.
https://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=8403077
Response to Hoenselaar from Pedersen et al
https://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=8479188
This Medscape interview with Dr. Christopher Ramsden is outstanding: https://www.medscape.com/viewarticle/780949_3
Excerpt:
“For a little perspective, if you eat a diet from foods that are raised naturally — it doesn’t matter if it is predominantly vegetable-based or meat-based — as everybody would have done up until about 100 years ago, you are going to consume something like 2%-3% of calories from linoleic acid. So with that perspective, the burden of proof would seem to be on the group advocating changes from what you would get in a natural diet, rather than vice versa.”
Axel,
did you see this, starting from around 7 min and onwards. From the recent AHA session:
https://www.youtube.com/watch?v=DFMtoafT70c&feature=player_embedded
“LDL is both necessary and sufficient for coronary disease.”
Darren K. McGuire tells about the prevailing perceptions about cholesterol from the UT Soutwestern Medical Center (The place where Brown & Goldstein do their job) and predicts that in the future other lipid-markers besides LDL will loose their significance. The new antibodies allow such a dramatic lipid-lowering that smoking, diabetes and high blood-pressure cannot no longer affect your CHD risk once you are treated. Moreover, the HDL-C levels will not be important anymore when the new injections enter the market. The quality of LDL will also be rendered largely insignificant when LDL is lowered drastically.
There’s not been a single case of atherosclerosis on the people with heterozygot PCSK9 knock-out mutation. These people have their life-time LDL-C around 50’s and 60’s (1,3-1,8mmol/l). Few individuals with homozygot PCSK9 mutation have been identified. These people have their life-time LDL extremely low (7-14mg/dl). They are all very healthy, no cognitive dysfunction, fully capable of reproduction, etc.
“But really, the real news is, that we shouldn’t really need these [statin] drugs. That for those of us who have normal genes, the reason why our blood is being filled up with cholesterol is because we’re basically eating too much cholesterol and too much animal fat. And if you look at populations where the diet is lower in cholesterol and fat, they don’t need these statin drugs; they have low cholesterols in their blood and they have twenty times lower rate of heart attacks than we do in the United States.”
Michael Brown, 2006
“The new antibodies allow such a dramatic lipid-lowering that smoking, diabetes and high blood-pressure cannot no longer affect your CHD risk once you are treated. Moreover, the HDL-C levels will not be important anymore when the new injections enter the market. The quality of LDL will also be rendered largely insignificant when LDL is lowered drastically.”
Could you pinpoint the exact time on the video when the abovementioned is mentioned? The point made around 16:30 was that in the context of lower and lower LDL levels, other risk factors become LESS RELEVANT.
Do your understand the difference between “less relevant” and “cannot affect”?
At the end of the discussion the one who interviews McGuire says something about we having come to a point where people can continue smoking and having high blood-pressure and yet be unaffected (from CHD). McGuire says jokingly “it was you, who said it”. I don’t have the time review the videos, but that was the way I understood it with my imperfect English.
So you took a joke as a recommendation?
That’s what I thought.
It wasn’t a joke. McGuire merely conveyd that he agreed, but did not dare to put it that bluntly. What I’ve understood, in English language the phrase “it was you, who said it” has a certain, specific meaning. It implies that the responder generally agrees but would not dare to exactly express him/her self with the same terms.
And here come the semantics.
There simply isn’t any basis to state the smoking or blood pressure will have no role in CVD prevention in the case of low LDL. Have a look at the risk charts for once.
Well, cigarette smoking becomes just “less relevant” at the presence of low LDL-C levels. We have a large body of ecologic evidence, especially from the Pacific region where low-fats diet are consumed and cigarette smoking is practiced en masse.
At the top of this, as McGuire concluded, not a single case of CHD among those with heterozygot PSCK9 knock-out mutation. Considering that the prevalence of this mutation is largely confined to the African-American minority, we can reckon that healthy-living patterns were probably even less prevalent among these individuals compared to the majority population. This assumption is of course not certain, but something one might easily expect on the basis of demographics.
Further evidence that omega-6 is problematic. Excerpt:
Another possible rationale for the differences in insulin resistance and inflammation across the diets may be the disparities in the linoleic content (C18:2),(11.4%, 9.4%, and 5.4% of total caloric intake for the 6%-SF, 12%-SF, and 24%-SF diets, respectively). Even when controlling for various ratios within an iso-caloric diet, the manipulation of one macronutrient, or sub-set of macronutrients, results in an uncontrollable alteration of another. As such, in the current study, alterations in the percentage of SF across diets also resulted in changes in the percentage of unsaturated fat. Thus, although the ratio of omega-6:omega-3 FAs was the same for each of the HFDs, the absolute quantity of linoleic acid in the 6%-SF and 12%-SF diets was greater than in the 24%-SF diet.
https://www.jlr.org/content/early/2012/10/28/jlr.M030700.abstract
Doc,
did you notice this. Kaiser Permanente has an important message for physicians. I forward this for you because you are interested in preventative medicine.
Nutritional Update for Physicians: Plant-Based Diets
https://www.thepermanentejournal.org/files/Spring2013/Nutrition.pdf
Thanks Richard. I agree. Plant based diets are certainly effective for cardiovascular prevention. The problem as I see it is that people/patients have such a hard time sticking to it.
Andre:
There’s nothing “industrial” in e.g. extra virgin olive oil – or if there is, then virtually everything we eat is “industrial”. And at any rate, “industrial” simply isn’t a synonym for “unhealthy” anymore than “natural” is a synonym for “healthy”.
As for Masterjohn’s article, it’s the same ol’ PUFA/n-6 scare. Nothing statistically sound – and compared to the results from the aforementioned meta-analyses, it’s … well, just what you might expect from a blog post from an author who has decided his stance & to hell with the evidence. Masterjohn has nothing solid to back up the notion that n-6 fatty acids/PUFA are detrimental as such. Therefore, he has to resort to
a) Discussing the irrelevant. The shortcomings of Mozaffarian et al under no circumstance negate the experimental data there is on the subject of PUFA & improved LDL receptor activity. This leads to the fact the since we have both the mechanism sorted out and scientific evidence that the replacement of SAFA with unsaturated fats does e.g. lower LDL and inflammatory response (metabolic ward data, RCT data on free-living subjects, epidemiological data – the whole bunch), it is indeed safe to conclude that the n-6/PUFA scare is nonsense.
b) Grasping at whatever he thinks can be interpreted as backing up his claims, e.g. rat studies, speculation on what MIGHT’ve happened had certain studies been carried out longer etc. etc.
The so-called “n-6/PUFA scare” is not a scare. Here’s the latest review:
Abstract
Although early studies showed that saturated fat diets with very low levels of PUFAs increase serum cholesterol, whereas other studies showed high serum cholesterol increased the risk of coronary artery disease (CAD), the evidence of dietary saturated fats increasing CAD or causing premature death was weak. Over the years, data revealed that dietary saturated fatty acids (SFAs) are not associated with CAD and other adverse health effects or at worst are weakly associated in some analyses when other contributing factors may be overlooked. Several recent analyses indicate that SFAs, particularly in dairy products and coconut oil, can improve health. The evidence of ω6 polyunsaturated fatty acids (PUFAs) promoting inflammation and augmenting many diseases continues to grow, whereas ω3 PUFAs seem to counter these adverse effects. The replacement of saturated fats in the diet with carbohydrates, especially sugars, has resulted in increased obesity and its associated health complications. Well-established mechanisms have been proposed for the adverse health effects of some alternative or replacement nutrients, such as simple carbohydrates and PUFAs. The focus on dietary manipulation of serum cholesterol may be moot in view of numerous other factors that increase the risk of heart disease. The adverse health effects that have been associated with saturated fats in the past are most likely due to factors other than SFAs, which are discussed here. This review calls for a rational reevaluation of existing dietary recommendations that focus on minimizing dietary SFAs, for which mechanisms for adverse health effects are lacking.
Full article: https://advances.nutrition.org/content/4/3/294.full
This review is behind the times and dismisses the meal studies on SFA totally.
Hi Reijo. Can you provide a reference so everybody understands what you mean by the meal studies on SFA.
Perhaps Reijo means the fact that SAFA is used when studying inflammation induced by fat content of food? It is the most pro-inflammatory of fats. See e.g.
https://www.ncbi.nlm.nih.gov/pubmed/21430255
https://www.ncbi.nlm.nih.gov/pubmed/19625064
https://www.ncbi.nlm.nih.gov/pubmed/22059644
As for Lawrence’s work, it has numerous problems.
1) The part “Dietary fatty acids and serum cholesterol” fails to point out the detrimental effect that SAFA has on LDL receptor activity. Nor does Lawrence mention the multitude of metabolic ward studies that show very clearly that the effect of SAFA on blood lipids is simply nothing to be excited about.
2) When discussing Ramsden et al (2010), Lawrence fails to consider the relevance of dose: the problems with PUFA & CVD seem to occur only when the intake is much, much higher than the average intake now. As for Mente et al (2009), Lawrence’s claim holds water only in context of separating n-3 fatty acids from the total picture, which is problematic and artificial.
3) Also, the points about red meat & its frequent use also smell of cherry-picking. Lawrence mentions the recent Micha et al (2010) meta-analysis, but points out ONLY that unprocessed red meat didn’t increase the risk of CAD events – leaving out the fact, that it DID increase the risk of strokes and cancer.
4) And, of course, the same ol’ story of inflammation and PUFA, in which he resorts to mostly rat studies – and leaves out the aforementioned fact that SAFA promotes inflammatory responses.
Meal studies are generally too problematic to connect SAFA directly to inflammation. Interview with Ron Krauss:
“Ron Krauss, there’s so much debate about saturated fat. Some studies say it’s perfectly fine to have it, and yet as a standard policy in most health clinics and with institutional recommendations, it’s, “keep the saturated fats low.” Meanwhile, your research has been a little frustrating. You don’t come out clearly saying one way or the other. Instead you say, it depends. It depends on what the saturated fat is eaten with.”
Krauss responds:
“I don’t know if I have to apologize for the way things really are, but that’s the way they are. And it’s not my fault. (laughs) My job is to help people understand how saturated fats work in the body, and explain it in the simplest possible way. We’re trying to work towards giving people that understanding, but simply the idea that saturated fat itself has an effect on health, outside the context of what one’s eating, is already a naive concept. Our studies are showing that how saturated fats interact with other foods is really more important than even we had realized when we started this work. And what we’ve learned about saturated fat, over the years, has shifted what we think. My first studies were driven by the hypothesis that lowering fat it good for you. This was in the old days, and I came out of that background of expecting lower fat diets to be healthier, and we wanted to study lower fats diets…We did our first studies in 1989, so it’s been over 20 years ago. I’m somewhat shocked to realize how long I’ve been doing this kind of work, but along the way we’ve learned things we didn’t expect to see. In fact for me the most important advances and the most interesting ones certainly are the ones that come out with opposite results from what you started with, where you expected one result and the results came out differently. And that happened when we first studied lower fat diets, thinking they would benefit the individuals who had higher heart disease risk, such as people who had the more dangerous, small particle LDL cholesterol, the pattern B profile, for example. What we found, to our surprise, initially, was that when we fed these low fat diets and reduced the fats by substituting carbohydrates, which was at that time and still remains the current paradigm, we really didn’t achieve what we had wanted to achieve. There was some improvement in the overall amount of cholesterol in the very small percentage of individuals who had very high amounts of small LDL particles in their blood already, but what was really astonishing to me at the time was that the majority of people we studied, the high percentage of people who had the normal metabolic profile, with more of the safer, Pattern A, larger particle LDL, a very large percentage of those people we studied actually shifted into the riskier, pattern B mode when we reduced their saturated fat intake.” https://www.meandmydiabetes.com/2012/04/17/ron-krauss-saturated-fat-red-meat-it-depends/
Read that last sentence carefully. A very large percentage of people with a normal metabolic profile (Pattern A, larger particle LDL) shifted into a riskier, pattern B mode when saturated fat intake was reduced. Notice also that all three studies cited by Mie above involved subjects with abnormal metabolic profiles. So where is the science that demonstrates that saturated fats promote inflammatory responses in people with normal metabolic profiles?
“Meal studies are generally too problematic to connect SAFA directly to inflammation. Interview with Ron Krauss:”
This interview doesn’t discuss meal studies nor metabolic ward studies.
“Read that last sentence carefully. A very large percentage of people with a normal metabolic profile (Pattern A, larger particle LDL) shifted into a riskier, pattern B mode when saturated fat intake was reduced.”
This may happen if fats, including saturated fat, is replaced with refined carbs. This has been shown multiple times. No such thing happens when SAFA is substituted by MUFA or PUFA nor carbs with low GI/high fiber content.
In addition, pattern B has clinical significance/correlates mostly with metabolic syndrome.
BTW, notice that later on Krauss goes to discuss that high SAFA & high red meat diet
caused clearly worse lipid profile.
“Notice also that all three studies cited by Mie above involved subjects with abnormal metabolic profiles. So where is the science that demonstrates that saturated fats promote inflammatory responses in people with normal metabolic profiles?”
Now Dave, care to explain why you consider that the inflammatory response of saturated fats would be (radically) different with people who have “normal” metabolic profiles?
I generally agree with David. The short term inflammation studies cited by Mie do not strongly support the conclusion that saturated fats are pro-inflammatory. In the first citation notice the weak conclusion and the last sentence –
“In summary, our data indicate that exchanging SFA from
butterfat for (n-6) PUFA in a mixed meal may decrease
postprandial lipemia and affects several postprandial markers
of inflammation and endothelial activation in overweight men.
Postprandial glucose and insulin concentrations were not
affected differently. The impact of these findings on long-term
health remains to be elucidated.”
In the second citation there was an increase in IL-6 after all meals but not CRP.
In the third citation there was no difference between meals together with respect to any of the markers. They seemed surprised that there were higher levels of NF-KB on the n-3 meal compared with the high safa meal. An interesting point made in this study is that some studies have shown that acute bouts of exercise acutely increase inflammation and oxidative stress, but chronically reduce systemic inflammation.
Regardless, these studies are way to short to conclude anything at all in my opinion.
IF you like to read what Ronald Krauss’s current view (2013) is on saturated fats, read this slide set named “setting the record straight the saturated fats”
According to him good fats are “Good (fats): Polyunsaturated fats (vegetable, nut, seed, fish oils)”
https://www.ciaprochef.com/wohf2013/presentations/2013WOHF_RonaldKrauss.pdf
And in this interview he recommends (conclusions and recommendations):
“Use oils high in polyunsaturated fat (e.g., safflower, corn oil) and avoid foods with trans fats from partially hydrogenated oils (read the label).
Unless you have a tendency for high cholesterol, you can *occasionally* eat cheese and butter.”
Also see this “In addition”, says Dr.Krauss, “the results add to the considerable body of evidence that consumption of diets high in both red meats and saturated fats should be kept to a minimum.”
https://www.chori.org/Current_News/2012/12_September_Krauss.html
This might also be of interest (about saturated fat and red meat)
https://goo.gl/ZbwLQ
And my slides on this study Krauss describes.
https://www.slideshare.net/pronutritionist/beef-low-carb-diet
But I’m sure this is not satisfying David or any others who provoke n-6 alarmism.
Thanks for the information. I’m not seeking satisfaction. Rather, I’m just trying to sort things out. As I learn, I alter my opinions according to what makes sense. At present, the world-wide increase in n-6 consumption seems to correlate more strongly with the increased burdens of aggression, depression, and non-communicable disease than saturated fat. Excerpt:
“…Crawford, Cordain, Simolopous, and others have advanced the concept of population-wide deficiencies of n-3 LCFAs in modern societies that rely on industrialized agriculture. Those authors cited, in part, that diets during the 4–5 million years of hominid evolution were likely abundant in seafood and other sources of n-3 LCFAs but had sparingly little contribution of calories from n-6-rich seed oils. In stark contrast, at the turn of the recent millennium, a single food source, soybean oil, appears to deliver 20% of all calories in the median US diet, with 9% of all calories from linoleic acid alone. To our knowledge, inadequate information is available to determine whether it is safe or unsafe to consume 9% of all calories as LA, a precursor to the pro-inflammatory arachidonic acid. One ecologic study indicated that greater intakes of LA from 1960 to 1999 in each of 5 countries predicted a 100-fold greater risk of homicide mortality. The increases in world LA consumption over the past century may be considered a very large uncontrolled experiment that may have contributed to increased societal burdens of aggression, depression, and cardiovascular mortality. Conversely, actively lowering LA intake must be carefully considered because of the large potential effects on agricultural economies. Alternative soybean variants or other sources of seed oils could be used to reduce LA intakes to levels currently seen in countries such as the Philippines, resulting in up to one-tenth lower estimated allowances for n-3 LCFAs as a percentage of energy. The limited worldwide fisheries and aquaculture production would be more likely to be able to meet world needs. Because LA constitutes such a large percent of calories in the US diet, it seems prudent to conduct large-scale intervention trials to determine whether lowering intakes can reduce cardiovascular risk and psychiatric morbidity. Increasing tissue concentrations of n-3 LCFAs on a population level may result in a substantial decrease in health care costs by reducing the illnesses that account for the largest burden of disease worldwide.
https://ajcn.nutrition.org/content/83/6/S1483.full.pdf+html
As for those RCTs that showed no inflammatory effects in humans with increased intake of linoleic acid, it’s important to realize that it takes a long time for linoleic acid to noticeably alter anything measurable in terms of inflammation. If linoleic acid were a fast-acting toxin, I would have died long ago. After I quit eating peanut butter in late 2009, it was several months before there was noticeable improvement in my health status. In the years since, I have regained a great deal of the strength and stamina I had lost in the previous decade due to high linoleic acid intake.
Interestingly, for someone who normally consumes very little linoleic acid, increasing intake can have a dramatic effect on metaboliic function. Susan Allport’s experience:
“My temporary shift to a high omega-6 diet didn’t result in any of these diseases (I hope), but I did experience an almost immediate thickening in my belly area. I know from the literature that omega-6s promote the development of fat tissue; still I was amazed it was happening to me. I didn’t weigh myself during the diet since I feared that any weight gain might send me on a secret, subversive diet, but I felt sure that I was putting on pounds. Any other symptoms were more fleeting and hard to attribute to diet alone. My stomach felt on fire after several meals, and I flushed more and longer after drinking alcohol. My husband thought my body smell was different; and I was short of breathe on several of my daily walks.
Flash forward 30 days, and I was thrilled to be at the end of my diet. By the time I returned to the University of Connecticut, I had a large, unpleasant wad of belly fat that I could grab in one hand. I was certain I had gained at least 5 pounds. I was shocked, therefore, when Dan picked me up in the morning, and then weighed me, before the follow up tests began. I couldn’t believe it when he said that my weight was exactly as it had been a month before – 56 kilograms or 124 pounds.”
https://www.susanallport.com/newsletter728511.htm
“As for those RCTs that showed no inflammatory effects in humans with increased intake of linoleic acid, it’s important to realize that it takes a long time for linoleic acid to noticeably alter anything measurable in terms of inflammation.”
Now Dave, once again: what is the experimental/physiological/etc. basis for this claim? How/why would LA be different from SAFA or trans fats?
In addition, this point from the clip above
“In stark contrast, at the turn of the recent millennium, a single food source, soybean oil, appears to deliver 20% of all calories in the median US diet, with 9% of all calories from linoleic acid alone.”
seems … shall I say, “odd”. According to NHANES 2009-10, a regular American gets about 6,5% of total energy intake from n-6 fatty acids. Now, the intake of LA cannot possibly be HIGHER than the entire PUFA intake, can it?
If linoleic acid would be toxic in long, it would show up in epidemiological studies. Linoleic acid intake is rather linked to decrease in inflammatory markers most cohorts. See Calder et al 2011 review paper I mentioned earlier. It’s free and has got nice table on this.
Nuts are very high in linoleic acid and they are either neutral or anti-inflammatory in RCTs. There is no solid human data to support your claims. Patient cases are interesting but not much of value when evaluating cause and effect.
Doc, some of the meal studies and longer RCT trials are referred in this slide deck of mine.
https://www.slideshare.net/pronutritionist/fats-and-inflammation
Mie mentioned also some more meal studies, but you may also look to a RCT by Bjermo et al. 2012.
https://www.ncbi.nlm.nih.gov/pubmed/22492369
I guess it all began from Cerillo’s work: see open access paper below (Fig 1). They used cream as “high fat”. As many know, OGTT is glucose load. Cream causes long and strong inflammatory response. OGGT causes to inflammation byt the effect is shorter.
https://circ.ahajournals.org/content/111/19/2518.full.pdf
I also want to point out that a recent systematic review on human RCTs (not meal studied) showed no inflammatory effects of n-6 fats in humans.
https://www.ncbi.nlm.nih.gov/pubmed/22889633
In conclusion, if anything, saturated fat is pro-inflammatory.
There’s not a single real diet-heart/lipid/atherosclerosis expert in the face of this planet who would promote the consumption of red meat, butter, high-fat cheese, whole milk & cream.
See this recent randomized trial. Robust CRP reducing effects (-~60 %) on PUFA and increase on SAFA (+~30 %, NS).
“C-reactive protein significantly decreased (p<0.02) after the PUFA diet period (6.61±2.49 vs. 2.56±1.64 mmol/l, p<0.05), whereas its increase after the SAFA diet did not reach statistical significance (2.95±1.98 vs. 3.30±2.54 mmol/l, n.s.)"
https://www.biomed.cas.cz/physiolres/pdf/62/62_145.pdf
Intakes of PUFA and SAFA were extremely high.
For me, it was frustrating to read this study due to lack of clarity. Quote:
“The result of this study proved that proportion of PUFA and SAFA the dietary intake might affect the systemic inflammation and to influence CVD risk in addition to a change of concentration of atherogenic lipoproteins.”
Then there’s this:
“Although the direct effect of SAFA in vivo in men has not been proven by epidemiology and clinical data definitely, the molecular biology mechanism of the proinflammatory effect of SAFA has been described at
the tissue cultures level (Suganami et al. 2009). Perhaps the reason for the incomplete proof of the direct effect of the SAFA diet on inflammation in humans to date is due to methodological problems.”
https://www.biomed.cas.cz/physiolres/pdf/62/62_145.pdf
The thing is, high saturated fat intake does not necessarily translate into high serum levels of saturated fat. Excerpt:
“One of the more remarkable results from Jeff Volek’s laboratory in the past few years was the demonstration that when the blood of volunteers was assayed for saturated fatty acids, those who had been on a low carbohydrate diet had lower levels than those on an isocaloric low-fat diet. This, despite the fact that the low-carbohydrate diet had three times the amount of saturated fat as the low-fat diet. How is this possible? What happened to the saturated fat in the low-carbohydrate diet? Well, that’s what metabolism does. The saturated fat in the low-carbohydrate arm was oxidized while (the real impact of the study) the low-fat arm is making new saturated fatty acid. Volek’s former student Cassandra Forsythe extended the idea by showing how, even under eucaloric conditions (no weight loss) dietary fat has relatively small impact on plasma fat.”
https://rdfeinman.wordpress.com/2012/02/22/saturated-fat-on-your-plate-or-in-your-blood/
An inflammatory effect stemming from high saturated fat intake has yet to be demonstrated in any sort of study or experiment involving humans.
In Dec 2013 it was shown that the inflammation is a product from Omega-6 and Fructose, Vegetable oils and Sugar. It is those two and LDL APOS B protein smaller than 25 nm that creates the plaque. Cholestrol have nothing to do with CVD. They are the medical care gang that are there to rebuild the damaged cells.
Sorry that trial is not RCT but non-controlled
RCTs are not the only legitimate science. Moreover, while RCTs may be helpful for determining whether a drug provides benefit or not, they may not be appropriate for determining whether nutrients such as saturated fats cause problems such as cancer or heart disease. More than 50 years of epidemiological studies and RCTs have done little to dispel the confusion over which fats cause harm at higher intakes and which are harmless or beneficial.
https://www.jclinepi.com/article/S0895-4356%2898%2900018-3/abstract
https://www.ncbi.nlm.nih.gov/pubmed/19364995
Context matters
The lower the fasting Triglyceride.
The much lower the after meal excursions in Blood fats , Sugars and Inflammation
” All other metabolic responses were independent of the P/S ratio ingested.”
https://www.ncbi.nlm.nih.gov/pubmed/18829281
.
Historically, Dietary Reference Values (DRV) for fats have been based on untested hypotheses as to how fats affect biochemistry. Recent research suggests that upper limits for total and saturated fat intake may be arbitrarily low. For example:
OBJECTIVE—The recommendations for dietary fats in patients with type 2 diabetes are based largely on the impact of fatty acids on fasting serum lipid and glucose concentrations. How fatty acids affect postprandial insulin, glucose, and triglyceride concentrations, however, remains unclear. The objective of this study was to study the effect of fatty acids on postprandial insulin, glucose, and triglyceride responses. https://care.diabetesjournals.org/content/30/12/2993.full
Another paper entitled “Changes in Serum Lipids and Blood Glucose in Non Diabetic Patients with Metabolic Syndrome after Mixed Meals of Different Composition”:
In conclusion, our study demonstrates that MetS+ patients respond with exaggerated triglyceridemia and insulinemia to meals that closely reflect in percent terms the habitual diet in our population and that moderate low-fat/high-CHO and low-CHO/high fat meals are associated with similar changes in serum TG, HDL-C, blood glucose, and plasma insulin in the postprandial period. Both low-fat and low-CHO diets can then be recommended in patients with MetS without risk of undesirable effects on postprandial lipemia and glycemia, provided that meals are largely based on fiber-rich, low glycemic index foods. Long-term studies demonstrated that both diets improve fasting lipoprotein profile in patients with MetS and are effective in reducing the prevalence of the components of the MetS [37, 38]. In a 5-month study [39], we found that both low-CHO and low-fat diets, similar to meals of the present study, were effective in improving clinical and biochemical determinants of MetS; however low-CHO diet was associated with a greater decrease of serum TG and blood pressure, whereas only low-fat diet was associated with a decrease of LDL-cholesterol. Then, the choice between one or the other diet may depend on the specific presentation of the syndrome. https://www.hindawi.com/journals/jnume/2012/215052/
So, to lower triglycerides and blood pressure, restrict carbohydrate intake. To lower LDL-cholesterol, consume a fiber-rich/low-fat diet.
Hello!
Are you aware there is an all natural supplement that reduces oxidative stress by an average of 40% after 30 days? Peer reviewed research found on http://www.pubmed.gov and multiple patents validate that fact.
It up-regulate “survival genes,” those genes that enable cells to survive in the face of stress from free radicals and other oxidants, and down-regulates other genes that promote inflammation and fibrosis to help the body function at an optimal level. Please let me know if you would like more info. It is truly a class creating breakthrough product!! (kablauwet@yahoo.com)
Chronic low-grade inflammation documented by high levels of inflammation-sensitive plasma proteins is associated with the fatality of future coronary events. Men who have been exposed to a low-grade inflammation many years earlier have a higher proportion of coronary heart disease deaths and less nonfatal myocardial infarction.