Estimated reading time: 9 minutes
Dapagliflozin (brand name Farxiga among others) and vericiguat are two very different and unrelated drugs that have recently been shown to be effective for patients with heart failure.
Of course, this is great news because heart failure is a common and often serious medical condition affecting an estimated 26 million people worldwide (1).
In the United States, 5.6 million individuals are estimated to have the condition, resulting in nearly $30 billion in healthcare-associated costs (2,3).
Also, despite several available treatment options, heart failure is often associated with severe disability, frequent hospitalizations, and dismal prognosis.
Currently, dapagliflozin is used to treat type 2 diabetes and, with certain restrictions, type 1 diabetes.
Vericiguat is still an unapproved drug, but will likely enter the U.S. market in 2021.
Let me start with a few words on heart failure. I will then review the recent data on the usefulness of these two drugs for the treatment of the disorder.
Heart Failure
Heart failure in itself is usually not considered to be a disease but rather a condition created by underlying heart disease. In many respects, advanced heart failure is synonymous with end-stage heart disease (4).
Coronary artery disease, high blood pressure, cardiomyopathy, myocarditis, heart valve disorders, obesity, diabetes, heart arrhythmia, and congenital heart defects are the most common causes.
In heart failure, the heart’s ventricles lose their ability to effectively fill with or pump blood, which results in increased fluid retention, dyspnea, fatigue, and organ damage.
The most common symptoms of heart failure are breathlessness (dyspnea), fatigue, exercise intolerance, and refractory volume overload leading to edema. Other symptoms include cough, lack of appetite, nausea, and swelling of the abdomen.
Heart failure is categorized into two subsets based upon the ability of the left ventricle to pump blood. This ability is assessed by measuring the left ventricular ejection fraction (LVEF).
Heart failure with reduced ejection fraction (HFrEF) is generally described as an LVEF of less than 40%, and heart failure with a preserved ejection fraction (HFpEF) is defined as an LVEF greater than 50%.
Patients with heart failure usually need diuretic drugs to relieve congestive symptoms. Furosemide is the most commonly used loop diuretic for the treatment.
In patients with HFrEF, treatment with ACE inhibitors, ARB’s or angiotensin receptor-neprilysin inhibitors (ARNI) is recommended. A beta-blocker should be added if tolerated. Aldosterone antagonists should be added on top of this treatment. All these drugs may improve prognosis and reduce the need for hospitalizations (6).
Dapagliflozin in Patients with Heart Failure?
Dapagliflozin is an SGLT2 inhibitor.
SGLT2 inhibitors inhibit the actions of a protein called sodium-glucose transporter 2. These drugs lower blood glucose by decreasing the reabsorption of glucose in the kidneys, thereby increasing urinary glucose excretion. In other words, the drugs lower blood glucose by throwing out excess glucose with the urine.
SGLT2 inhibitors also reduce blood pressure and cause weight loss.
In one study, another SGLT2 inhibitor, empagliflozin (Jardiance) significantly reduced the risk of death from cardiovascular causes, death from any cause, and the risk of hospitalization for heart failure in people with type 2 diabetes (5)
The DAPA-HF Trial
The results of the DAPA-HF trial were published in the New England Journal of Medicine in November 2019 (6). Unfortunately, these important data were largely overlooked by many because of the imminent COVID-19 pandemic.
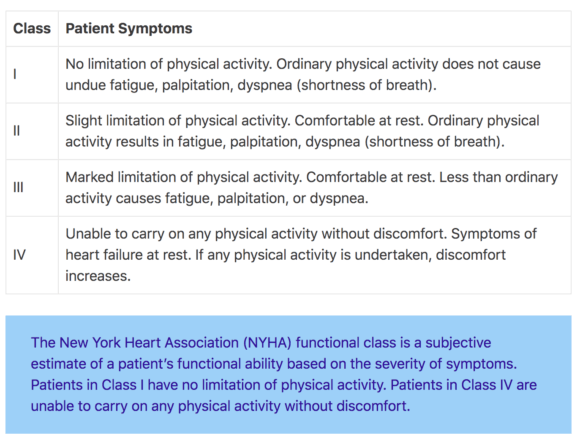
In the DAPA-HF trial, 4744 patients with New York Heart Association class II, III, or IV heart failure and an ejection fraction of 40% or less were randomized to receive either dapagliflozin (at a dose of 10 mg once daily) or placebo. The study drug was added on top of recommended therapy which, based on current clinical guidelines, involves many drugs.
The primary outcome of the DAPA-HF trial was a composite of worsening heart failure (hospitalization or an urgent visit resulting in intravenous therapy for heart failure) or cardiovascular death.
Over a median of 18.2 months, the primary outcome occurred in 386 of 2373 patients (16.3%) in the dapagliflozin group and in 502 of 2371 patients (21.2%) in the placebo group (hazard ratio, 0.74; 95% confidence interval [CI], 0.65 to 0.85; P<0.001).
A first worsening heart failure event occurred in 237 patients (10.0%) in the dapagliflozin group and in 326 patients (13.7%) in the placebo group (hazard ratio, 0.70; 95% CI, 0.59 to 0.83).
Death from cardiovascular causes occurred in 227 patients (9.6%) in the dapagliflozin group and in 273 patients (11.5%) in the placebo group respectively (hazard ratio, 0.82; 95% CI, 0.69 to 0.98).
There were 276 deaths from any cause (11.6%) in the dapagliflozin group and 329 (13.9%) in the placebo group (hazard ratio, 0.83; 95% CI, 0.71 to 0.97).
The main conclusion is that among patients with heart failure and a reduced ejection fraction, the risk of worsening heart failure or death from cardiovascular causes is lower among patients who receive dapagliflozin than among those who receive placebo, regardless of the presence or absence of diabetes.
Why Is a Diabetic Drug like Dapagliflozin Helpful in Patients with Heart Failure?
A total of 55% of patients in the DAPA-HF trial had type 2 diabetes while 45% had not. Dapagliflozin was as effective in patients without type 2 diabetes as in those with diabetes.
This demonstration provides support for the hypothesis that the treatment has beneficial actions other than glucose lowering. Indeed, the results of the trial suggest that the the therapeutic role of SGLT2 inhibitors extends beyond patients with diabetes.
Multiple mechanisms have been proposed to explain the benefits of dapagliflozin in patients with heart failure (7).
SGLT‐2 inhibitors may help preserve kidney function which may be extremely important for patients with heart failure.
The drugs also appear to reduce the loading condition of the heart muscle, including both preload and afterload. These effects are partly mediated through increased diuresis.
SGLT-2 inhibitors may also provide alternative cardiac energy supply for heart muscle cells in the form of cardiac ketones.
They may also reduce or reverse cardiac injury, reduce left ventricular mass, and improve endothelial dysfunction.
Both systolic and diastolic function of the left ventricle may be improved through these mechanisms.
Although the underlying mechanisms have not been exactly outlined, it is clear that SGLT-2 inhibitors are not only helpful for the treatment of diabetes but important ammunition in the treatment of heart disease.
Vericiguat in Patients with Heart Failure?
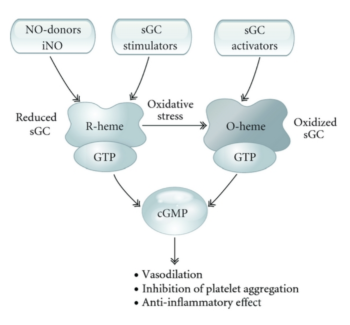
Vericiguat is a soluble guanylate cyclase (sGC) stimulator. sGC is a key enzyme of the nitric oxide (NO) signaling pathway,
Upon binding of NO to a prosthetic heme group on sGC, the enzyme catalyzes the synthesis of the second messenger cyclic guanosine monophosphate (cGMP), which produces vasodilatation, inhibits smooth muscle proliferation, leukocyte recruitment, and platelet aggregation.
For many years, impaired NO and cGMP signaling have been implicated in the development of cardiovascular disease.
Organic nitrates that target the NO signaling pathway have been used to treat cardiovascular disease for more than 150 years (9). More recently, gaseous NO administered by inhalation has been approved for the treatment of several conditions.
Nonetheless, these agents have several important limitations. Cardiovascular disease is associated with resistance to NO and organic nitrates and the long-term efficacy of organic nitrates is limited by the development of tolerance.
Hence, recent efforts have aimed at identifying pharmacological agents that could target sGC-cGMP signaling directly.
Vericiguat enhances the cGMP pathway by directly stimulating sGC through a binding site independent of nitric oxide, and it sensitizes sGC to endogenous nitric oxide by stabilizing nitric oxide binding to the binding site (8).
The VICTORIA Trial
Acute worsening of heart failure is a common cause for hospital admissions and is associated with a three-month mortality rate of 7% to 11% after discharge (9).
The Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction (VICTORIA) assessed the efficacy and safety of vericiguat in patients with a reduced ejection fraction and chronic heart failure with recent decompensated heart failure.
Hence, patients enrolled in the VICTORIA trial were at high risk of hospitalization and cardiovascular death following a recent heart failure decompensation.
The results of the study were published in The New England Journal of Medicine on March 28, 2020 (10).
The study assigned 5050 patients with chronic heart failure (New York Heart Association class II, III, or IV) and an ejection fraction of less than 45% to receive vericiguat (target dose, 10 mg once daily) or placebo, in addition to guideline-based medical therapy. The primary outcome was a composite of death from cardiovascular causes or first hospitalization for heart failure.
Over a median of 10.8 months, a primary-outcome event occurred in 897 of 2526 patients (35.5%) in the vericiguat group and in 972 of 2524 patients (38.5%) in the placebo group (hazard ratio, 0.90; 95% confidence interval [CI], 0.82 to 0.98; P=0.02).
A total of 691 patients (27.4%) in the vericiguat group and 747 patients (29.6%) in the placebo group were hospitalized for heart failure (hazard ratio, 0.90; 95% CI, 0.81 to 1.00).
Death from cardiovascular causes occurred in 414 patients (16.4%) in the vericiguat group and in 441 patients (17.5%) in the placebo group (hazard ratio, 0.93; 95% CI, 0.81 to 1.06).
The composite of death from any cause or hospitalization for heart failure occurred in 957 patients (37.9%) in the vericiguat group and in 1032 patients (40.9%) in the placebo group (hazard ratio, 0.90; 95% CI, 0.83 to 0.98; P=0.02).
Based on the absolute risk reduction, the number needed to treat with vericiguat for 1 year to prevent a primary-outcome event is approximately 24 patients
The main conclusion of the study is that among patients with high-risk heart failure, the incidence of death from cardiovascular causes or hospitalization for heart failure is lower among those who receive vericiguat than among those who receive placebo.
As expected, symptomatic hypotension and syncope were more common in the patients receiving vericiguat than in those receiving placebo. At the initial 16-week follow-up visit, the hemoglobin level was slightly lower in the patients receiving vericiguat than in those receiving placebo, as previously reported for this class of agents.
The Bottom-Line
The DAPA-HF trial showed that the SGLT‐2 inhibitor dapagliflozin (brand name Farxiga among others), a drug initially approved for the treatment of diabetes, was effective in patients with heart failure and reduced ejection fraction.
When added on top of guideline-recommended therapy, the drug reduces the risk of worsening heart failure and improves survival.
Dapagliflozin is contraindicated in patients with symptomatic hypotension or systolic blood pressure <95 mmHg, kidney disease (estimated glomerular filtration rate (eGFR) <30 mL per minute per 1.73 m2), or rapidly declining renal function.
The VICTORIA trial showed that among patients with high-risk heart failure, vericiguat (a soluble guanylate cyclase (sGC) stimulator) reduced the incidence of death from cardiovascular causes and hospitalizations for heart failure compared to placebo.
These two recently published clinical trials may provide a significant shift in the current treatment of heart failure.


I wonder at the definition of T2D in the DAPA-HF trial; Dr. Joseph Kraft’s work describes diabetes in situ prior to increased blood glucose. Could the SGLT2 inhibitor be working to the benefit of those patients not yet diagnosed with T2D? Thanks for your attention to this trial in these times. Unfortunately, other maladies have not stopped.
Thanks Paula.
You make an interesting point.
The issue of patients with insulin resistance or prediabetes is not discussed in the original publication.
Food for thought.